Costly protease inhibitors work well in many patients, but call for careful monitoring
Thomas Reinke
Contributing Editor
In less than a year, two new protease inhibitors — telaprevir (Incivek) and boceprevir (Victrelis) — have changed the standard of care for hepatitis C by introducing a new mechanism of action, but their significance goes beyond that.
They are the first new medications for the disease in 10 years and they have brought dramatic improvements in outcomes by knocking out this infection in many more patients, including previous poor responders. They have also complicated care by adding a third agent to the previous standard regimen of two agents.
The new agents are examples of how therapy management for new and costly medications is moving from traditional utilization and outcomes management to more sophisticated strategies. The focus on adherence and medication possession rates is giving way to patient selection criteria and close monitoring of clinical results.
Approximately 3.9 million people in the United States have chronic hepatitis C virus (HCV) infection, and about 5,000 acute cases surface annually.
Boceprevir and telaprevir are direct-acting agents that block the growth of viruses by directly disrupting essential viral functions. They were approved only as supplements to the previous standard treatment, a combination of ribavirin (Rebetol, Copegus) plus peginterferon alfa-2a (Pegasys) or peginterferon alfa-2b (PegIntron).
Both are also approved only for use in one subset of patients: genotype 1 patients. HCV has at least 6 genotypes and 50 different subtypes, with genotype 1 being the most common.
In trials, the new drugs increased cure rates, known as sustained viral response (SVR) rates, by 30 to 40 percentage points over control groups receiving peginterferon and ribavirin. SVR means that no HCV is detected in the blood.
For telaprevir in one study, a SVR at 24 weeks was achieved in 75 percent of patients, compared with 44 percent for control patients. For boceprevir, in one study the success rate for triple therapy was 66 percent, compared with 38 percent for control patients.
Based on these results, the American Association for the Study of Liver Diseases updated its clinical guidelines to recommend triple therapy with a protease inhibitor, interferon, and ribavirin as the standard of treatment for hepatitis C.
“The hope for improved outcomes produced an immediate demand for these medications,” says David Lassen, PharmD, chief clinical officer of Prime Therapeutics, a pharmacy benefit manager. “Previously the utilization of medications used to treat hepatitis C was on the decline, causing an overall decrease in the pharmacy cost trend over time. With the entrance of these two new agents, we have seen a significant increase in drug trend driven by increased use and cost.”
Doubling the cost of HCV management
An article in the November 2011 issue of the Journal of Managed Care Pharmacy by Prime’s pharmacy experts says that the new regimen will more than double the cost of HCV management. Telaprevir costs $49,200 for its 12-week regimen. The required peginterferon and ribavirin add $17,175 for 24 weeks and $34,349 for 48 weeks of therapy.
Boceprevir costs $26,410 for a 24-week duration and $35,213 for 32 weeks. Peginterferon and ribavirin add $20,037 or $34,349 to the total.
Complexities
The protease inhibitors add to the complexity of treatment for hepatitis C. Poor response and anemia were problems with the previous peginterferon-ribavirin regimen.
Anemia has increased significantly with the new agents. In studies of telaprevir it increased from 17 percent to 36 percent, and in studies of boceprevir it increased from 20–30 percent to 45–50 percent. The increased anemia may stimulate more frequent therapy with erythropoiesis-stimulating agents, further driving up costs.
The new agents have many drug interactions, including conflicts with statins, and there are new side effects, such as the skin rash that is associated with telaprevir. This is in addition to flu-like symptoms that are associated with peginterferon.
There are other implications for the new medications. Both have demonstrated success and are approved for use in poor responders, so the demand for the new agents may spike until this cohort is resolved.
The new regimen requires monitoring of viral loads at specific points, and there are parameters for discontinuing therapy when treatment is futile.
Lassen says that Prime Therapeutics planned ahead to be able to handle the protease inhibitors. “In anticipation of the approvals we took a step back to revise our management strategies to be sure we could capitalize on the value of these medications in an evidence-based manner,” he says.
Prime Therapeutics made significant changes in its utilization and therapy management activities. It expanded the patient-specific data it captures. For initial authorizations, it asks about comorbidities such as HIV/AIDS and about the extent of liver disease. It also asks about previous treatments with protease inhibitors for HCV.
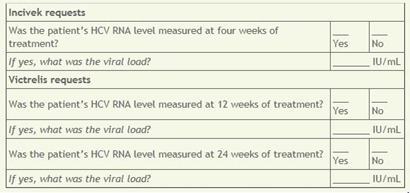
For renewing authorizations, the company is going further. “We are looking at the viral load component to ensure that the therapy is on track because some of the patients are not naïve to treatment,” says Lassen. Providers are asked to report the specific HCV RNA levels from lab tests at 4 or 12 weeks, depending on which new drug is being used.
While Prime, like other PBMs and even health plans, is interested in capturing more clinical data to improve its quality and cost management, Lassen emphasizes that this has to be done appropriately. “We were careful and thoughtful in our approach. Any request for additional information as part of prior authorization or utilization management can have a negative impact on providers.”
He adds: “It is also important that requests like lab results as part of renewals do not interrupt therapy. There can be legitimate delays in getting tests done, and we make it a point to provide continued coverage while tests are being arranged.”
For health plans
The protease inhibitors are game changers in the treatment of hepatitis C — the first new medications in 10 years with a new mechanism of action.
“Triple therapy is complex, “Lassen says. “Patients cannot be switched to a different agent once a course of therapy is started, and it is critical that therapy be completed. Up front, you need a clear set of criteria for patient selection, and then there needs to be close monitoring and a clear set of supportive care activities as patients move through therapy.”
How well did that new drug work?
Health plans and PBMs are collecting more patient-specific clinical data as part of their prior-authorization and therapy management. These data are increasingly important in ensuring the appropriate use of new, more complex, targeted medications.
The questions below are asked by Prime Therapeutics, a pharmacy benefit manager owned by several Blue Cross Blue Shield plans, when a renewal authorization is being considered for telaprevir (Incivek) and boceprevir (Victrelis). HCV RNA results indicate the level of hepatitis C infection and the progress of therapy.
The questions are taken from the prior-authorization form used by Blue Cross Blue Shield of Texas.
No comments:
Post a Comment