From Seminars in Liver Disease
JasonGrebely, B.Sc., Ph.D.; Gregory J.Dore, M.B.B.S., Ph.D., F.R.A.C.P., M.P.H.
Posted: 02/01/2012; Semin Liver Dis. 2011;31(4):331-339. © 2011 Thieme Medical Publishers
Abstract and Introduction
Abstract
The burden of hepatitis C virus (HCV)-related morbidity and mortality continues to rise. Progression to advanced liver disease among HCV-infected individuals generally requires decades, but we are entering an era where those infected with HCV in the 1970s and 1980s are at significant risk of mortality. Liver disease has overtaken drug-related harm as the major cause of mortality in HCV-infected individuals in many settings. Direct-acting antiviral therapies have provided renewed optimism, but HCV treatment uptake will need to increase markedly to reduce liver disease mortality. This review provides updated information on the natural history of HCV, disease-specific causes of mortality among people with HCV, estimates and projections of HCV-related disease burden and mortality and individual and population-level strategies to reduce mortality. The considerable variability in mortality rates within subpopulations of people with HCV will be outlined, such as in people who inject drugs and those with HIV co-infection.
Introduction
The next decade will be a crucial period in the public health response to hepatitis C virus (HCV) infection. The rapid development of direct-acting antiviral (DAA) therapy for chronic HCV infection has brought considerable optimism to the HCV sector,[1] with the realistic hope that therapeutic intervention will soon be more effective and offer shorter treatment duration. The initial phase of combination pegylated interferon (PEG-IFN), ribavirin, and one or more DAA agents will be associated with increased toxicity and complexity of therapeutic management,[1] but over the course of this decade, strategies including interferon-free regimens with enhanced tolerability, dosing schedules, and simplified monitoring protocols should emerge.
These therapeutic advances are urgently required, as a high HCV incidence 20 to 30 years ago is now reflected in a growing burden of advanced HCV-related liver disease.[2–8] Without effective therapeutic intervention, the projected liver disease burden will continue to rise in many countries,[9–14] for at least the next one to two decades, and beyond in those settings that have experienced ongoing high-level HCV transmission.
Despite the prospect of greatly improved therapies, the challenges ahead for HCV infection are considerable. HCV treatment uptake is very low in many countries[12,15,16] and within marginalized subpopulations in countries with higher treatment uptake.[16–20] The explanations for low uptake are multifactorial[21] and not the focus of this review, but interferon-related toxicity, lack of HCV treatment infrastructure, suboptimal government subsidization programs and medical insurance coverage, as well as competing patient health and social priorities are likely to remain as contributing factors in the near future.
An improved understanding of morbidity and mortality among people with HCV infection will guide clinical management and therapy decision-making, both at the individual patient and population strategic levels. This review will provide updated information on the natural history of HCV infection, disease-specific causes of mortality among people with HCV infection, estimates and projections of HCV-related disease burden and mortality, the potential impact of HCV treatment on disease burden, and individual and population-level strategies to reduce mortality. The considerable variability in mortality rates within subpopulations of people with HCV will be outlined, and a particular focus given to the issue of competing mortality risk among people who inject drugs and those with human immunodeficiency virus (HIV) co-infection.
Natural History of Chronic HCV Infection
An estimated 75% of people who acquire HCV infection progress to development of persistent of chronic HCV infection,[22] with associated risk of progressive liver disease, cirrhosis, liver failure, or hepatocellular carcinoma.[23] The remaining 25% of people achieve spontaneous HCV clearance;[22] however, these individuals may be reinfected in the setting of ongoing HCV exposure. Although many of those with reinfection undergo subsequent spontaneous viral clearance, others develop persistent infection.[24–30]
As reviewed elsewhere,[31] the risk of HCV-related liver disease morbidity and mortality depends on several factors: (1) the duration of HCV infection;[32–34] (2) the presence of cofactors for development of liver fibrosis (such as male gender,[35–37] ethnicity,[38,39] older age at infection,[37,40–42] heavy alcohol intake,[43–45] HIV[46–49] or chronic hepatitis B virus (HBV) co-infection,[50,51] diabetes,[52,53] obesity,[54,55] and hepatic steatosis[56,57]); (3) access to HCV therapy and a favorable treatment response;[58] and (4) competing mortality risk (such as HIV[7,49] and illicit drug-related overdose[2,6,7,8,49]). The generally slowly progressive nature of chronic HCV, with limited advanced liver disease in the initial 10 to 15 years of infection (even in those individuals with cofactors for fibrosis development), means that duration of HCV infection and its surrogate, age, are key determinants of mortality risk.[31] Thus, a 50-year-old individual with 30 years chronic HCV is likely to have a higher HCV-related mortality risk, even in the absence of liver disease cofactors, than a 30-year-old individual with 5 to 10 years infection and several cofactors. However, the 50-year-old individual with 30 years infection, with heavy alcohol intake, obesity, and regular cannabis smoking (recently shown to be a liver fibrosis cofactor[59]) will be at particularly high risk.
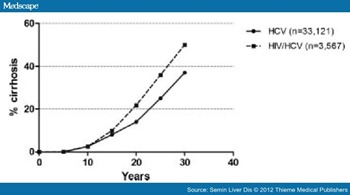
The risk of HCV-related cirrhosis based on duration of infection has recently been estimated through large systematic reviews of disease progression studies in HCV mono-infected and HIV/HCV co-infected populations (Fig. 1).[33,34] The exponential relationship between duration of infection and cirrhosis relates to the generally protracted disease course (few very fast progressors), the cumulative nature of cirrhosis prevalence (even linear rates of progression lead to a nonlinear/upward curve for cirrhosis), and the potential for more rapid fibrosis progression at older age.
Figure 1. Risk of hepatitis C virus- (HCV-) related cirrhosis based on duration of infection as estimated through large systematic reviews of disease progression studies in HCV mono-infected and human immunodeficiency virus (HIV)/HCV co-infected populations.33,34
Without therapeutic intervention, an estimated 7 to 18% of HCV mono-infected individuals will develop cirrhosis over a 20-year infection period,[31,34] and be at considerable risk of HCC (1–6% per annum) or liver failure (2–3% per annum).[31] Thus, a significant minority of people with chronic HCV (possibly 10–20%) are likely to have shortened life expectancy through HCV-related mortality. A further large proportion will have HCV-related morbidity with reduced quality of life.[60]
Causes of Mortality Among People With HCV Infection
The distribution of causes of death within a population with a chronic disease will depend on several factors: (1) disease-specific natural history and mortality risk, (2) distribution of duration of chronic disease within the population, (3) access to effective therapeutic intervention that alters natural history, and (4) age distribution and competing mortality risk within the population.
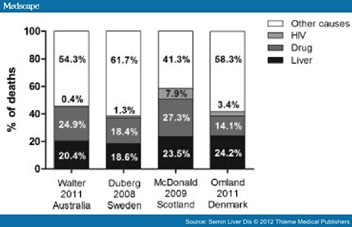
The three major disease-specific groupings for mortality among people with HCV infection are drug-related, liver disease-related, and HIV-related. Drug-related mortality includes drug overdose and suicide. Liver disease-related mortality includes decompensated cirrhosis and HCC. The mortality distribution based on these major groupings in population-based HCV notification—death registry linkage studies in Australia (New South Wales),[8] Sweden,[6] Scotland[7] and Denmark[2] (Lars Omland, personal communication, August 11, 2011) is shown in Fig. 2. In these four countries, the proportion of liver disease-related deaths varied from 19 to 24%, and for drug-related from 18 to 27%. A high proportion of drug-related mortality is consistent with injection drug use (IDU) being the major mode of HCV acquisition in all four settings. The proportion of HIV-related deaths was highest in Scotland (7.9%)[7] where 4% of the HCV-notified population was HIV co-infected, and lowest in Australia (0.4%)[8] where only 0.5% was HIV co-infected. Settings in which the HIV co-infection rate is even higher than Scotland, such as in developed countries in North America and Europe, would be expected to have larger proportions of deaths related to HIV disease.
Figure 2. Contribution of human immunodeficiency virus- (HIV-) related, liver-related, drug-related, and other cause-related mortality (percentage of total number of deaths) in large population-based studies of people diagnosed with HCV infection in Australia (New South Wales),8 Sweden,6 Scotland,7 and Denmark2 (Lars Omland, personal communication, August 11, 2011).
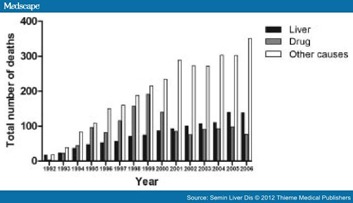
Temporal trends in mortality rates and distribution among people with HCV infection are clearly important to monitor. From 1992 to 2006 in New South Wales, Australia, there has been a steady increase in the number of people with HCV dying from liver-related causes (Scott Walter, personal communication, August 11, 2011) (Fig. 3). In contrast, the number of deaths from drug-related causes increased rapidly during the 1990s, but has declined since 1999 due to the well-documented heroin "drought" starting in late 1999 and its likely subsequent reductions in IDU. The number of liver disease-related deaths reflects the expanding pool of chronic HCV including larger numbers with prolonged duration of infection (aging cohort effect). From 1997 to 2006 the age-adjusted liver disease mortality rate was stable (around 15 deaths per 10,000 person years), indicating no impact of improved HCV therapy.[8] The lack of an effect of HCV treatment on individual risk of liver-disease mortality probably relates to the generally low-treatment uptake rate, suboptimal efficacy (particularly among those with advanced liver disease), and the relatively short period of follow-up since improvements.
Figure 3. Total number of deaths due to liver-related, drug-related, and other causes in a large population-based study of people diagnosed with hepatitis C virus (HCV) infection in New South Wales, Australia, from 1992 to 2006 (Scott Walter, personal communication, August 11, 2011).
Consistent with increasing numbers of people with HCV dying from liver-related causes, the proportion of all liver disease deaths with underlying HCV is increasing in many settings, as demonstrated in a population-based study in Scotland.[4] The burden of HCV-related advanced liver disease is also seen in increasing numbers of HCV-related liver transplants in many countries.[16]
Mortality Among Injection Drug Users With HCV Infection
The prevalence of HCV among regular IDUs and people receiving opioid substitution therapy (OST) is 60 to 80%;[61] thus, mortality studies in these populations are likely to reflect mortality among IDUs with HCV infection. Overall, mortality rates in these two populations are 1 to 2 per 100 person years,[62,63] although there is evidence that OST reduces drug-related mortality.[64,65] A study among the OST population in Australia demonstrated a considerably higher drug-related mortality rate compared with liver-disease mortality, with the ratio varying from threefold when receiving OST to 18-fold when not receiving OST.[65] However, this study covered a period during the 1990s with very high rates of drug-related mortality. A more recent mortality linkage study in New South Wales that included people on OST in 1980 to 1984 has demonstrated increasing rates of liver disease mortality, which in recent years is the leading cause of death overtaking drug-related mortality.[66] This study is of great importance as it demonstrates the impact of liver disease on mortality within an aging cohort, particularly when rates of IDU decline.
In Canada, the Vancouver community-based CHASE cohort (81% and 42% have used illicit and injection drugs in the past 6 months; HCV prevalence is 64%) has also examined all-cause and liver-related mortality through data linkage to a death registry.[49] Between 2003 and 2007, the rate of mortality was 1.9 per 100 person-years, with causes of death being 7% liver-related, 20% drug-related, 21% HIV-related, and 52% other cause-related. All-cause mortality was associated with age >50 years and HIV infection. Further, those >50 years of age were at significant risk of liver-related mortality. Given that many communities of IDUs were infected with HCV in the 1970s and 1980s, there will inevitably be greater incidence of liver disease over the next decade.
The potential future burden of advanced liver disease within aging cohorts is also reflected in an autopsy study among individuals dying from opioid toxicity in New South Wales, Australia.[67] Among 841 deaths over a 5-year period (1998–2002), the HCV prevalence was 71% and cirrhosis was present in 7%.[67] However, in those aged >44 years at death (n = 75), cirrhosis prevalence was 25%.
Mortality in Other Populations With HCV Infection
Injection drug use has been the major mode of HCV acquisition in North America, Europe, and Australia. However, in other settings, HCV transmission has largely been through non-IDU modes and the contribution of drug-related mortality is therefore considerably reduced. Thus, the impact of HCV-related disease on mortality rates and distribution is more evident.
In one study from the United Kingdom, 924 individuals who had acquired HCV infection via blood transfusion were traced during a look-back program.[68] By the end of 2004, 28% had died (255 of 924), with 26% dying of liver-related causes. The risk of liver-related mortality in those with HCV infection was three times higher than the control group of anti-HCV negative transfusion recipients. In Taiwan, 23,785 persons (aged 30 to 65 years, HCV prevalence 4.5%) were recruited from seven townships between 1991 and 1992 and followed through 2004.[69,70] Among participants with HCV mono-infection (n = 1,040), 171 died by 2004; 28% of deaths were liver-related. After adjusting for gender, age, cigarette smoking, and alcohol consumption, those with HCV mono-infection were two times more likely to die of any cause and five times more likely to die of chronic liver disease and cirrhosis compared with those without HCV. These data suggest that HCV still leads to excess mortality when drug-related and HIV effects are removed.
Mortality in People With HIV/HCV Co-infection
As reviewed elsewhere,[71] co-infection with HIV decreases spontaneous clearance of HCV infection,[72] increases HCV RNA levels,[73] increases HCV-related liver disease progression,[46,74] and reduces response to IFN-based therapy.[75,76] Since the introduction of triple-combination antiretroviral therapy in the mid-1990s, overall mortality rates among HIV-infected populations have declined dramatically.[77] Further, the distribution of causes of death have altered considerably, with a declining proportion of acquired immunodeficiency syndrome- (AIDS-) related mortality and increasing proportions of cardiovascular and liver disease mortality.[78,79] The contribution of liver disease to mortality is particularly high in settings with a high HIV/HCV co-infection prevalence;[78] however, even in Australia where only 10 to 15% of people with HIV are HCV co-infected, liver disease contributes to 11% of deaths (Kathy Petoumenos, personal communication, August 12, 2011).
Within the HIV/HCV co-infected population, factors that influence rates and distribution of mortality are access to antiretroviral therapy, access to and effectiveness of HCV therapy, drug use, and age distribution.[71] In Australia, within the HIV/HCV co-infected population there is universal access to antiretroviral therapy and high levels of uptake, relatively limited regular ICU (the vast majority are men who have sex with men), and an aging population. Among HIV/HCV co-infected patients enrolled in the Australian HIV Observational Database (AHOD; n = 3,531), liver disease has been the underlying cause in 26% of deaths (14 of 55 deaths) as compared with only 9% of deaths (16 of 181) in those with HIV alone (Kathy Petoumenos, personal communication, August 12, 2011).
Further, although the lifespan of those with HIV infection has been improved through the availability of contemporary antiretroviral therapy, the lives of those with HCV/HIV co-infection remain much shorter.[71] In Denmark, one study compared the mortality rates of 3,990 HIV-infected persons and the general population.[80] The study demonstrated that although mortality has dropped significantly in HIV-infected persons (from a high of 124 per 1000 person years in the era preceding HIV antiviral therapy to 25 per 1000 person years in 2000–2005), the impact was less pronounced among those co-infected with HCV (57 per 1000 person-years in those with HCV/HIV vs 19 per 1000 person-years among those with HIV alone). In a large study of 23,441 HIV-infected persons (76,893 person-years of follow-up), the frequency of and risk factors associated with liver-related deaths were assessed (66% with HCV co-infection, 17% active HBV co-infection).[78] Among 1246 deaths (5.3%; 1.6 per 100 person-years), liver-related death was the most frequent cause of non-AIDS related death (14.5% were from liver-related causes). Predictors of liver-related deaths were latest CD4 cell count, older age, IDU, HCV infection, and active HBV infection. Given the underreporting of liver-related disease, the actual impact is probably even greater.[71]
The Potential Impact of Improving HCV Therapy on Mortality Rates
The burden of HCV-related advanced liver disease is projected to increase further in many countries.[9,10,11,12,13,14] A major public health issue is the potential impact of improving HCV therapy on these projected increases in mortality. In HIV, the availability of effective combination antiretroviral therapy from the mid-1990s dramatically reduced the overall mortality rate and altered the distribution of causes of death (increasing proportion of non-AIDS related deaths).[78–80] The lower overall disease-specific HCV mortality risk compared with HIV and more protracted disease progression (life expectancy for chronic HCV infection is on average reduced by several years rather than decades for HIV) mean that a more-effective and more broadly implemented therapeutic intervention will be unable to have the dramatic impact that was seen for antiretroviral therapy. However, there is considerable potential for improved HCV therapeutic intervention to alter expected HCV-related mortality, particularly when the temporal "ageing cohort" effect is most pronounced.
The recent introduction of DAA therapy in combination with PEG-IFN and ribavirin will enhance treatment response rates for those with chronic HCV genotype 1 infection and shorten duration of therapy for many patients.[1] However, low HCV treatment (PEG-IFN/ribavirin) uptake rates for those with chronic HCV genotype 2/3,[12,15–20] despite treatment success of 70 to 80% and shorter duration therapy (generally 24 weeks), suggest that initial DAA-based response rate improvements will have a modest impact at the population level. Recent evidence suggests that IFN-free combination DAA therapy with high rates of treatment response is feasible.[81] Improvements in DAA therapy tolerability and dosing schedules are highly likely given agents in phase II/III development.[1] Large population-level impacts on HCV-related liver disease mortality will likely require IFN-free combination therapy that is tolerable, has a favorable dosing schedule, and is effective over a relatively short duration. Such HCV therapeutic advances are within reach over the next decade.
How can we Currently Prevent People With Hcv Infection From Dying?
The availability of PEG-IFN/ribavirin-free regimens for the treatment of HCV infection are still 5 to 10 years away and other strategies will be required if we are to stem the projected rise in liver-disease burden.[9–14] Strategies that increase the proportion of individuals diagnosed, assessed, and treated for HCV infection with currently available treatment regimens are required.
Increasing the number diagnosed with HCV infection will be important as we move forward. In the United States, the true number of people infected with HCV is likely underestimated (5.2 million as compared with previous estimates of 3.3 million from household surveys), given that homeless people, prisoners, IDUs, and other marginalized populations at high-risk of HCV are often not included in national household surveys.[82] Strategies to enhance diagnosis of HCV may include the promotion of national HCV testing guidelines,[83] and enhanced education and training of general practitioners about HCV testing and diagnostic criteria to enhance diagnosis and referral. Further strategies include the provision of mentoring diagnosis programs among general practitioners with higher case loads of HCV-infected patients,[84] an improved awareness of programs offering comprehensive multidisciplinary HCV care (particularly for IDUs), and improved pathways for referral. Incorporation of HCV assessment and treatment services into drug and alcohol treatment settings is also required.
Enhancing the proportion assessed for HCV is crucial. Non-invasive tests of fibrosis (e.g., FibroScan and FibroTest) offer considerable opportunities for enhanced screening and assessment of liver disease. In a study at one hospital in France, a cohort of 1457 consecutive patients with chronic HCV were assessed for liver fibrosis by liver biopsy, FibroScan, FibroTest, aspartate aminotransferase to platelet ratio index (APRI), and FIB-4 score to evaluate all-cause and liver-related mortality during a 5-year follow-up period.[85] Survival was significantly decreased among patients diagnosed with severe fibrosis (regardless of the noninvasive method employed) and all noninvasive methods were able to predict shorter survival times, although FibroScan and FibroTest had higher predictive values. These tools will help physicians determine prognosis at earlier stages and therefore allow enhanced targeting of therapy to those with significant liver disease.
Strategies are needed to enhance HCV assessment and treatment in the community to reduce mortality among people with HCV. Barriers to expanding HCV treatment in the community are multifactorial and include issues of access to therapy and barriers at the level of the patient, practitioner, and system.[86] HCV-infected patients often have complex social, medical, and psychiatric comorbidities, complicating decisions around care. Currently, there is limited infrastructure for the provision of HCV assessment and treatment delivery beyond well-established, hospital-based liver clinics. However, successful strategies to improve engagement with HCV services and enhance HCV assessment have been explored.[21] One model to enhance access to HCV care for underserved populations focused on the integration of community-based health centers in New Mexico using state-of-the-art telehealth technology to provide training and support for primary care providers to deliver best-practice HCV care.[87] This model was effective, with similar responses to HCV treatment observed among community-based clinics as compared with a university-based hospital.[87] This approach represents a needed change from the conventional approaches in which specialized care and expertise are concentrated in academic medical centers in urban areas.
Lastly, given that 70 to 80% of current HCV infections occur among IDUs,[88] it is clear that strategies to reduce mortality among those living with HCV will require specific strategies for this marginalized group. There is now overwhelming evidence that the treatment of HCV infection in this population is safe and effective across multiple models of care.[89] As such, older IDUs in particular will be an important group to follow clinically (perhaps with noninvasive liver fibrosis screening) and perhaps offer intensified HCV assessment and treatment in an effort to reduce liver-related mortality.
Conclusion
Our understanding of morbidity and mortality among people with HCV infection has greatly improved over the past several decades. In large population-based studies, liver-related and drug-related causes of death account for approximately one-half of all deaths (one-quarter each) among people with HCV infection. Liver disease burden continues to rise in many countries,[9–14] particularly given the low HCV treatment uptake in many countries[12,15,16] and subpopulations.[16–20] Although novel HCV treatments (particularly PEG-IFN/ribavirin-free regimens) offer great hope for reducing the future mortality associated with HCV, combinations with improved tolerability and shorter duration are still 5 to 10 years away. Current efforts will need to focus on enhancing the diagnosis, assessment, and treatment of HCV-infected patients at greatest risk of liver disease progression to reduce mortality among those living with HCV infection.
References
- Thompson A, Patel K, Tillman H, McHutchison JG. Directly acting antivirals for the treatment of patients with hepatitis C infection: a clinical development update addressing key future challenges. J Hepatol 2009;50(1):184–194
- Omland LH, Jepsen P, Krarup H, et al; DANVIR Cohort Study. Increased mortality among persons infected with hepatitis C virus. Clin Gastroenterol Hepatol 2011;9(1):71–78
- El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis 2011;53(2):150–157
- McDonald SA, Hutchinson SJ, Bird SM, et al. The growing contribution of hepatitis C virus infection to liver-related mortality in Scotland. Euro Surveill 2010;15(18):19562
- Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet 2006;368(9539):938–945
- Duberg AS, Törner A, Davidsdóttir L, et al. Cause of death in individuals with chronic HBV and/or HCV infection, a nationwide community-based register study. J Viral Hepat 2008;15(7):538–550
- McDonald SA, Hutchinson SJ, Bird SM, et al. A population-based record linkage study of mortality in hepatitis C-diagnosed persons with or without HIV coinfection in Scotland. Stat Methods Med Res 2009;18(3):271–283
- Walter SR, Thein HH, Amin J, et al. Trends in mortality after diagnosis of hepatitis B or C infection: 1992–2006. J Hepatol 2011;54(5):879–886
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27(9):1485–1491
- Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980–2004. Hepatology 2008;48(1):137–145
- Bosetti C, Levi F, Lucchini F, Zatonski WA, Negri E, La Vecchia C.Worldwide mortality from cirrhosis: an update to 2002. J Hepatol 2007;46(5):827–839
- Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 2009;50(6):1750–1755
- NCHECR. Epidemiological and economic impact of potential increased hepatitis C treatment uptake in Australia. Sydney, Australia: National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales; 2010
- Werb D, Wood E, Kerr T, Hershfield N, Palmer RW, Remis RS. Treatment costs of hepatitis C infection among injection drug users in Canada, 2006–2026. Int J Drug Policy 2011;22(1):70–76
- Lettmeier B, Mühlberger N, Schwarzer R, et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J Hepatol 2008;49(4):528–536
- NCHECR. HIV, viral hepatitis, and sexually transmissible infections in Australia Annual Surveillance Report. Sydney, Australia: National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales; 2010
- Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health 2008;33(3):126–133
- Grebely J, Genoway K, Khara M, et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy 2007;18(5):437–443
- Grebely J, Bryant J, Hull P, et al. Factors associated with specialist assessment and treatment for hepatitis C virus infection in New South Wales, Australia. J Viral Hepat 2011;18(4):e104–e116
- Strathdee SA, Latka M, Campbell J, et al; Study to Reduce Intravenous Exposures Project. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis 2005;40(Suppl 5):S304–S312
- Grebely J, Dore GJ. An expanding role for primary care providers in the treatment of hepatitis C virus infection in the community. Hepatology 2011. In press
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006;13(1):34–41
- Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002;36(5, Suppl 1):S35–S46
- Aitken CK, Lewis J, Tracy SL, et al. High incidence of hepatitis C virus reinfection in a cohort of injecting drug users. Hepatology 2008;48(6):1746–1752
- Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet 2002;359(9316):1478–1483
- Micallef JM, Macdonald V, Jauncey M, et al. High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. J Viral Hepat 2007;14(6):413–418
- Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010;138(1):315–324
- Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009;200(8):1216–1226
- Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology 2006;44(5):1139–1145
- van de Laar TJ, Molenkamp R, van den Berg C, et al. Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. J Hepatol 2009;51(4):667–674
- Seeff LB. The history of the ''natural history'' of hepatitis C (1968–2009). Liver Int 2009;29(Suppl 1):89–99
- Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001;34(4 Pt 1):809–816
- Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008;22(15):1979–1991
- Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48(2):418–431
- Kenny-Walsh E; Irish Hepatology Research Group. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med 1999;340(16):1228–1233
- Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in Germany: a 20-year multicenter study. Hepatology 2000;32(1):91–96
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349(9055):825–832
- Wiley TE, Brown J, Chan J. Hepatitis C infection in African Americans: its natural history and histological progression. Am J Gastroenterol 2002;97(3):700–706
- Sterling RK, Stravitz RT, Luketic VA, et al. A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans. Clin Gastroenterol Hepatol 2004;2(6):469–473
- Vogt M, Lang T, Frösner G, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med 1999;341(12):866–870
- Casiraghi MA, De Paschale M, Romano` L, et al. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology 2004;39(1):90–96
- Minola E, Prati D, Suter F, et al. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood 2002;99(12):4588–4591
- Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology 1998;27(6):1730–1735
- Harris DR, Gonin R, Alter HJ, et al; National Heart, Lung, and Blood Institute Study Group. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med 2001;134(2):120–124
- Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol 2005;3(11):1150–1159
- Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001;33(4):562–569
- Ragni MV, Belle SH. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J Infect Dis 2001;183(7):1112–1115
- Thomas DL, Shih JW, Alter HJ, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis 1996;174(4):690–695
- Grebely J, Raffa JD, Lai C, et al. Impact of hepatitis C virus infection on all-cause and liver-related mortality in a large community-based cohort of inner city residents. J Viral Hepat 2011;18(1):32–41
- Pontisso P, Gerotto M, Benvegnu` L, Chemello L, Alberti A. Coinfection by hepatitis B virus and hepatitis C virus. Antivir Ther 1998;3(Suppl 3):137–142
- Gaeta GB, Stornaiuolo G, Precone DF, et al. Epidemiological and clinical burden of chronic hepatitis B virus/hepatitis C virus infection. A multicenter Italian study. J Hepatol 2003;39(6):1036–1041
- Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology 2002;36(3):729–736
- Ratziu V,Munteanu M, Charlotte F, Bonyhay L, Poynard T; LIDO Study Group. Fibrogenic impact of high serum glucose in chronic hepatitis C. J Hepatol 2003;39(6):1049–1055
- Hourigan LF, Macdonald GA, Purdie D, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology 1999;29(4):1215–1219
- Ortiz V, Berenguer M, Rayón JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol 2002;97(9):2408–2414
- Leandro G, Mangia A, Hui J, et al; HCV Meta-Analysis (on) Individual Patients' Data Study Group. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology 2006;130(6):1636–1642
- Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 2001;33(6):1358–1364
- Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol 2010;8(3):280–288, 288, e1
- Hézode C, Roudot-Thoraval F, Nguyen S, et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 2005;42(1):63–71
- Hsu PC, Federico CA, Krajden M, et al. Health utilities and psychometric quality of life in patients with early- and latestage hepatitis C virus infection. J Gastroenterol Hepatol 2011Epub ahead of print
- Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378(9791):571–583
- Degenhardt L, Hall W, Warner-Smith M. Using cohort studies to estimate mortality among injecting drug users that is not attributable to AIDS. Sex Transm Infect 2006;82(Suppl 3):iii56–iii63
- Degenhardt L, Bucello C, Mathers B, et al. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 2011;106(1):32–51
- Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O'Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction 2008;103(3):462–468
- Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug Alcohol Depend 2009;105(1–2):9–15
- Gibson A, Randall D, Degenhardt L. The increasing mortality burden of liver disease among opioid-dependent people: cohort study. Addiction 2011;106(12):2186–2192
- Darke S, Kaye S, Duflou J. Comparative cardiac pathology among deaths due to cocaine toxicity, opioid toxicity and non-drug-related causes. Addiction 2006;101(12):1771–1777
- Harris HE, Ramsay ME, Andrews NJ; HCV National Register Steering Group. Survival of a national cohort of hepatitis C virus infected patients, 16 years after exposure. Epidemiol Infect 2006;134(3):472–477
- Iloeje UH, Yang HI, Jen CL, et al; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus Study Group. Risk and predictors of mortality associated with chronic hepatitis B infection. Clin Gastroenterol Hepatol 2007;5(8):921–931
- Yang HI, Chen CJ, Jen CL, et al. Causes of death associated with hepatitis B or hepatitis C virus infections in a long-term population-based cohort study. Paper presented at: Digestive Disease Week 2007; May 19–24, 2007; Washington, DC
- Thomas DL, Leoutsakas D, Zabransky T, Kumar MS. Hepatitis C in HIV-infected individuals: cure and control, right now. J Int AIDS Soc 2011;14:22
- Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 2000;284(4):450–456
- Thomas DL, Astemborski J, Vlahov D, et al. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis 2000;181(3):844–851
- Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood 2002;100(5):1584–1589
- Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al; APRICOT Study Group. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351(5):438–450
- Hadziyannis SJ, Sette H Jr, Morgan TR, et al; PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140(5):346–355
- Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet 2010;376(9734):49–62
- Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166(15):1632–1641
- Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 2001;32(3):492–497
- Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007;146(2):87–95
- Gane EJ, Roberts SK, Stedman CA, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2010;376(9751):1467–1475
- Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int 2011; Epub ahead of print
- Australian Government Department of Health and Ageing. Hepatitis C Subcommittee of the Ministerial Advisory Committee on AIDS SHH, the Blood Borne Virus and Sexually Transmissible Infections Subcommittee of the Australian Population Health Development Committee. National hepatitis C testing policy. Canberra: Australian Government Department of Health and Ageing; 2007
- Treloar C, Newland J, Harris M, Deacon R, Maher L. A diagnosis of hepatitis C - insights from a study on patients' experiences. Aust Fam Physician 2010;39(8):589–592
- Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140(7):1970–1979, 1979, e1–e3
- Grebely J, Bryant J, Hull P, et al. Factors associated with specialist assessment and treatment for hepatitis C virus infection in New South Wales, Australia. J Viral Hepat 2011;18(4):e104–e116
- Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011;364(23):2199–2207
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005;5(9):558–567
- Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis 2009;49(4):561–573
No comments:
Post a Comment