Gilead Sciences, Inc.
333 Lakeside Drive
Foster City, CA 94404
Tel: (650)522-6009
Fax: (650)522-5260
Poster
Number 1421
47th Annual Meeting of the
European Association for the Study of the Liver
April 18 - 22, 2012
Barcelona, Spain
M. Sulkowski1, M. Rodriguez-Torres2, E. Lawitz3, M. Shiffman4, S. Pol5, R. Herring6, J.G. McHutchison7, P.S. Pang7, K.A. Wong7, B. Massetto7, Y. Zhu7, D.M. Brainard7, D. Wyles8, F. Habersetzer9
1Johns Hopkins University School of Medicine, Lutherville, MD; 2Fundacion de Investigacion de Diego, Santurce, PR; 3Alamo Medical Research, San Antonio, TX; 4Liver Institute of Virginia, Richmond, VA; 5Hôpital Necker, Paris, France; 6Nashville Gastrointestinal Specialists, Inc.,
Nashville, TN; 7Gilead Sciences, Inc., Foster City, CA; 8University of California, San Diego, La Jolla, CA; 9Hôpitaux Universitaires de Strasbourg, Strasbourg, France
Introduction
- Combinations of direct-acting antivirals (DAAs) without interferon (IFN) have demonstrated variable rates of sustained virologic response (SVR) in genotype 1 patients1,2
─ Lower SVR rates have been reported in genotype 1a as compared to genotype 1b HCV
─ The optimal duration of IFN-free treatment regimens in genotype 1 has not been established
─ Virologic failure in IFN-free regimens without a nucleos(t)ide analogue has been associated with multidrug resistance - This ongoing Phase 2 study (NCT01353248) was designed to assess the effi cacy and safety of a 3-DAA-containing regimen plus ribavirin (RBV), including a protease inhibitor, NS5A inhibitor, and a non-nucleoside NS5B inhibitor
- We report preliminary SVR4, SVR12, and safety data from patients treated for 12 or 24 weeks
Table 1. In Vitro Characteristics of DAAs Administered in the Current Study
*In vitro and following 3 or 7 days of monotherapy in HCV-infected patients
Methods
Patients
Major inclusion criteria:
- Chronic infection with HCV genotype 1a or 1b
- HCV treatment-naïve
- Plasma HCV RNA ≥104 IU/mL during screening
- Exclusion of cirrhosis by liver biopsy within 2 years or Fibroscan within 6 months
Major exclusion criteria:
- Coinfection with human immunodefi ciency virus or hepatitis B virus
- Contraindication to treatment with IFN and/or RBV
- Current or prior hepatic decompensation
- Prespecifi ed laboratory abnormalities
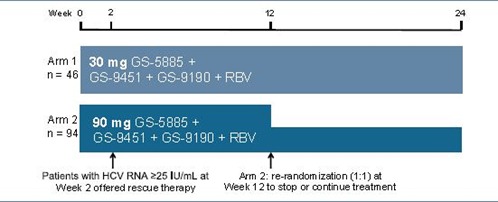
Study design (Figure 1)
- Patients were randomized 1:2 to the following treatment groups:
─ Arm 1: GS-5885 30 mg QD + GS-9451 200 mg QD + GS-9190 30 mg BID + RBV
─ Arm 2: GS-5885 90 mg QD + GS-9451 200 mg QD + GS-9190 30 mg BID + RBV - Patients with HCV RNA ≥25 IU/mL at Week 2 (non-vRVR) were offered peginterferon (PEG)-containing rescue therapy or discontinuation from the study
- Patients in Arm 2 with HCV RNA <25 IU/mL from Week 2 through Week 10 were
re-randomized at Week 12 to either stop treatment or continue treatment through Week 24
- Randomization stratifi ed by HCV RNA at screening (≤ or >800,000 IU/mL) and genotype 1a or 1b
- Virologic breakthrough defi ned as confi rmed, on-treatment HCV RNA ≥25 IU/mL after Week 2
─ Patients with breakthrough offered PEG-containing rescue therapy - Plasma HCV RNA measured using the Roche COBAS TaqMan HCV/HPS assay v2.0 with a lower limit of quantifi cation of 25 IU/mL
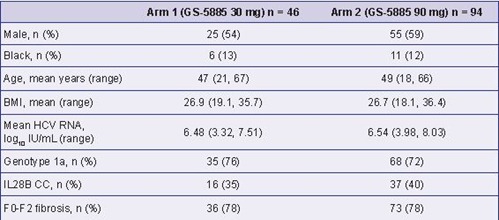
Table 2. Summary of Baseline Characteristics (N = 140)
Figure 2. Patient Disposition
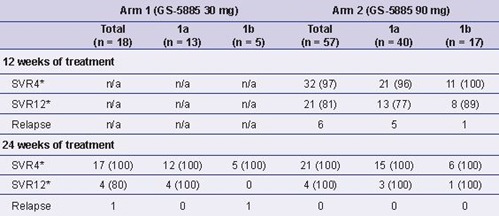
Table 3. Preliminary Post-treatment Response Rates by Treatment Arm and
Genotype Subtype, n (%)
*SVR4 and SVR12 rates calculated using treatment completers who had available data at the given timepoint
Figure 3. On-treatment Response by Arm, IL28B Genotype, and HCV Subtype
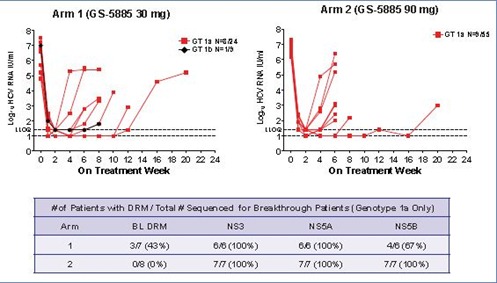
Figure 4. HCV RNA Kinetics in Patients with Virologic Breakthrough by Treatment Arm and Genotype Subtype
45 patients enrolled in rescue Rescue therapy with PEG + GS-5885 + GS-9451
Table 4. Patients Achieving HCV RNA <25 IU/mL During Rescue Therapy
a. 1 early termination (ET) due to lack of effi cacy; 1 ET due to an AE
b. ET due to lack of effi cacy; 1 of 16 patients experienced breakthrough after achieving <25 IU/mL
c. 1 ET due to lack of effi cacy; 1 ET due to an AE; 1 patient has not reached <25 IU/mL after 16 weeks in rescue
Table 5. Safety Summary, n (%)
- One subject in Arm 1 had 2 SAEs (pancreatitis requiring overnight hospitalization and viral gastroenterititis); no doses of study medications were missed
- Two subjects in Arm 2 had 5 AEs (acute psychosis, alcohol intoxication, decreased muscle mass, heartburn, irritability)
- Reported grade 3 AEs were pancreatitis (also SAE), stomatitis, fatigue, elevated bilirubin, viral gastroenteritis (also SAE), alcohol poisoning (led to study discontinuation), tendonitis, and acute psychosis
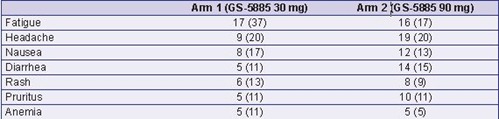
Table 6. Most Common (≥10%) Treatment-Emergent AEs, n (%) Arm 1 (GS-5885 30 mg) Arm 2
Table 7. Laboratory Parameters of Interest, n (%)
- Rates and severity of anemia were consistent with what has been reported in other IFN-free, RBV-containing studies3,4
- Indirect hyperbilirubinemia was observed in approximately 60% of patients without concomitant transaminase elevations
─ GS-9451 is an inhibitor of the bilirubin transporter protein OATP1B1 and has been associated with transient indirect hyperbilirubinemia in healthy volunteers5
─ No Grade 4 (≥6.0 g/dL) hyperbilirubinemia was observed
Summary and Conclusions
Preliminary data from this ongoing Phase 2 study of a multi-DAA
regimen without a nucleos(t)ide analogue has shown in genotype 1
patients that:
- A regimen including 3 DAAs + RBV was well tolerated for up to 24 weeks
- The 90-mg dose of GS-5885 provides improved antiviral effi cacy over the 30-mg dose of GS-5885 without an increase in toxicity
- Rates of viral breakthrough and relapse were lower in genotype 1b patients than in genotype 1a patients
- Patients with the IL28B CC genotype showed lower rates of breakthrough than IL28B non-CC patients, particularly in Arm 2 (GS-5885 90mg)
- Virologic breakthrough was associated with multi-DAA resistance
─ Viral suppression with the addition of PEG occurs in most virologic failures
References and Acknowledgements
1. Lok A, et al. N Engl J Med 2012;366;3:216-24.
2. Zeuzem S, et al. Gastroenterology 2011;141:2047-54.
3. Zeuzem et al. AASLD 2011. Abstract LB-15
4. Gane EJ, et al. AASLD 2011. Abstract 34
5. Yang JC, et al. EASL 2012.
The study team and investigators thank the patients for their participation in this clinical trial.
© 2012 Gilead Sciences, Inc.






No comments:
Post a Comment