Liver International
Early View (Online Version of Record published before inclusion in an issue)
Patrick Ingiliz1,*, Jürgen K. Rockstroh2
Article first published online: 17 APR 2012
DOI: 10.1111/j.1478-3231.2012.02796.x
© 2012 John Wiley & Sons A/S
Abstract
With the licensing of the first hepatitis C (HCV) protease inhibitors (PI), telaprevir (TVR) and boceprevir (BOC), cure rates for chronic HCV infection will substantially improve. Human immunodeficiency virus- chronic hepatitis C (HIV-HCV) co-infected patients are in urgent need for these new drugs, because they are facing both severe liver disease and lower response rates than HCV monoinfected patients. The currently available efficacy data are however, limited to two phase II trials. Fortunately, TVR and BOC appear to be able to improve cure rates in co-infected patients. A major challenge for clinicians will be the management of drug–drug interactions of antiretroviral drugs and new PI. As HCV PI are also metabolized by the cytochrome P450 3A4 system interactions are probable as well with non-nucleoside reverse transcriptase inhibitors as with HIV PI. To our knowledge, TVR can only be safely used with one protease inhibitor, boosted atazanavir, and also with efavirenz (EFV), although this combination requires TVR dose adjustments. Boceprevir should not be combined with HIV PI and should not be combined with EFV. The approval of TVR and BOC will create new chances of cure also for HIV-HCV co-infected patients. However, the decision who to treat or not has to be taken carefully on the basis of fibrosis stage and previous treatment outcomes. In addition, HIV therapy needs to be optimized according to the available drug–drug interaction data.
New directly acting agents (DAAs) have appeared on the horizon for the treatment of chronic hepatitis C (HCV) infection and numerous agents are currently tested in clinical phase II and III. Agents targeting the active site of the non-structural (NS) 3/4A HCV protease are the foremost developed. These drugs can be divided into two major groups: linear and macrocylic protease inhibitors (PI). With the first two linear PI [telaprevir (TVR) and boceprevir (BOC)] licensed in the US and Europe in May and July/September 2011, respectively, sustained virological response (SVR) rates are expected to rise in HCV monoinfected genotype 1 patients up to 75% in treatment naïve[1, 2] and over 60% in treatment experienced patients [3, 4].
Of the 33 million people currently living with the human immunodeficiency virus (HIV) worldwide around 20% (∼7 million) are chronically co-infected with hepatitis C. This population is mainly represented by individuals with a past history of intravenous drug use, haemophiliacs and recipients of contaminated blood [5]. Moreover, outbreaks of acute hepatitis C among HIV-positive men who have sex with men have been reported in several large European and North American cities since the year 2000 [6, 7, 8] making HCV an ongoing sexually transmitted disease in this setting. With the introduction of a combined antiretroviral therapy (ART), life expectancy for HIV-infected patients has dramatically improved and hepatitis C co-infection has emerged as an important factor of non-AIDS morbidity and mortality in HIV patients [9, 10].
The current standard of care for chronic HCV in co-infected patients is a 24–72 weeks treatment course with pegylated interferon and ribavirin, leading to an overall cure rate of roughly 50% in a Western European patient population [11, 12]. Factors associated with response to HCV treatment are HCV genotype, baseline HCV viral load, viral kinetics during treatment, CD4 percentage, insulin resistance, age, obesity, fibrosis stage and, more recently described, the host's interleukin 28 B (IL28B) genotype [12, 13]. The HIV infection adversely affects all phases of the natural history of chronic hepatitis, increasing the frequency of viral persistence after acute infection, the level of viremia among persistently infected persons, the rate of progression to cirrhosis and the proportion of persons who will ultimately develop end-stage liver disease [14, 15, 16, 17, 18], although this may account less for patients with a preserved immune function [19].
This accelerated course of HCV disease in HIV co-infected patients strengthens the need for new treatment options in this setting: HIV-HCV co-infected patients have lower response rates than monoinfected patients to standard therapy [11] and many patients will eventually die from end-stage liver disease if they remain untreated.
Treatment of HIV/HCV co-infected patients with the new PIs clearly is accompanied by some additional challenges. HCV viral load on average is one log higher than that in HCV monoinfected patients potentially increasing the risk for resistance development. In addition, adherence will be challenged by taking HCV drugs on top of the underlying HIV therapy with a huge potential for drug–drug interactions. In HIV-HCV co-infected patients, only two phase II trials with TVR and boceprevir have been conducted to date and final results have yet not been presented. The approval of TVR and boceprevir in 2011 will force HIV physicians to decide whether to use these new agents in co-infected genotype-1 patients or not. Non-response to DAAs, however, may induce resistance mutations and even lead to drug class resistance [20]. This article aims to review the currently available data on efficacy and drug–drug interactions and to offer a structured approach to the use of DAAs in HIV-HCV co-infected patients with HCV genotype 1.
Directly acting agents in Human immunodeficiency virus-chronic hepatitis C co-infected patients
The mechanism of HCV (NS3) protease inhibition involves formation of a stable reversible covalent bond between the ketoamide of the drug and the NS3 protease active site serine, which is the protease that mediates the cleavage of the HCV polyprotein to form the functional proteins essential for viral propagation [21].
As there is evidence that they share similar metabolic routes [22], interactions between HIV PIs and HCV PIs are likely: Telaprevir is a substrate and inhibitor of the cytochrome P450 3A system. Pharmacokinetic studies have shown a decline of TVR areas under the curve (AUC) when co-administered with boosted atazanavir (ATV), lopinavir (LPV), darunavir (DRV) and fosamprenavir (fAMP). Indeed with lopinavir/r TVR AUC dropped to 46% suggesting that these two drugs should not be combined as the low TVR exposure may increase the risk for resistance emergence in the setting of potentially subtherapeutic TVR drug levels. The lowest AUC decline (20%) was seen with boosted ATV [23]. Therefore, boosted ATV was the only PI/r which was studied in the ongoing phase II trial with TVR. The AUCs of DRV/r and fAMP/r declined considerably (40% and 47%) under co-administration of TVR, whereas ATV/r and LPV/r remained stable. The observed reductions of almost half of the respective drug concentrations of the antiretroviral agents bear the potential for HIV treatment failure and eventually drug resistance development so that again the co-administration of these particular PIs and TVR is currently not recommended.
As the enzyme inductor efavirenz (EFV) leads to reduction of TVR levels [24], TVR doses have to be increased to 1125mg q8h when these drugs are co-administered.
Raltegravir (RAL) is not metabolized by the CYP3A4 system, and the co-administration of RAL with TVR did not affect TVR pharmacokinetics in healthy volunteers, and RAL exposure was increased by 31% [25].
The metabolism of boceprevir (BOC) seems to be more complex, as the drug is also a substrate of the aldo-keto reductase, as well as a substrate and inhibitor of the cytochrome P450 3A system and a substrate and inhibitor of the P-glycoprotein membrane pumps. Pharmacokinetic studies did not show an impact on BOC when co-administered with low-dose ritonavir (RTV) in healthy volunteers [26]. Therefore, relevant drug–drug interactions between BOC and boosted HIV PI were considered rather unlikely and all corresponding boosted PIs as well as maraviroc and RAL were allowed to be used in the ongoing phase II study in co-infected patients. BOC trough levels (minimal concentrations), however, decreased by over 40% in combination with EFV [26] raising the question whether this could be clinically meaningful, and NNRTIs were not allowed in the phase II study. Recently published DDI studies however, have shown that BOC coadministration reduces the exposure of ATV/r, LPV/r and DRV/r by 35%, 34%, and 44%, respectively, and trough concentrations by 48%, 43%, and 59%, respectively. BOC AUC decreased by 45% and 32% when coadministered with LPV/r and DRV/r, respectively, but stayed stable when coadministered with ATV/r (27). As expected, co-administartion with raltegravir did not show significant interaction patterns in healthy volunteers [28].
In addition to the outlined interactions between the newly available HCV protease inhibitors and HIV drugs it is important to remember that clinical relevant interactions exist between HIV NRTIs and ribavirin which partially compete for phosphorylation and also can have overlapping adverse event profiles. During PegINF plus ribavirin therapy, ddI is contraindicated in patients with cirrhosis and should be avoided in patients with less severe liver disease because of an increased risk for hepatic decompensation and lactic acidosis. D4T and AZT should also be avoided because of overlapping toxicities (weight loss, lactic acidosis and anaemia, respectively) if possible. Abacavir can be safely used with concomitant HCV therapy if appropriate ribavirin dosages (weight adapted) are being used.
Besides direct interactions with antiretrovirals, both HCV PIs show comprehensive interaction patterns with other drugs commonly used in HIV-infected patients [24, 29]: The antimycobacterial agents rifampicin or rifabutin reduce BOC and TVR drug levels and co-administration is contraindicated. The same applies to the antidepressant St. Johns wort. The PDE-5-inhibitor sildenafil is often used in the setting of HIV infection and treatment to relieve erectile dysfunction; the co-administration with BOC or TVR, however, is contraindicated because of an increased risk of sildenafil adverse reactions such as hypotension or collapse. Methadone plasma levels may increase or decrease with BOC and decrease with TVR. However, a drug–drug interaction study of TVR and methadone showed a reduction in total R-methadone concentrations during TVR co-administration, but as the unbound concentration of R-methadone was unchanged, it was not considered clinically relevant [30]. The impact of this interaction needs to be assessed in clinical practice.
Efficacy and side effects
Preliminary results (SVR 12) from the two phase II trials in HIV-HCV co-infected patients have been presented at several conferences during the year 2011 and 2012 [31, 32, 33].
As of now, study 110 compared 60 HIV-HCV co-infected patients treated either with triple therapy [pegylated interferon alpha 2a (Peg2a), ribavirin (RBV) and TVR] or with standard of care (SOC: Peg2a, RBV).
Patients were stratified in an antiretroviral treatment (ART) naïve arm (A), or an ART arm (B) where they received tenofovir (TDF) and emtricitabin (FTC) or lamivudine (3TC) with either EFV or boosted ATV/r. Telaprevir was given orally at a dosage of 750 mg TID in ART naïve or in ATV/r-receiving patients, and at a dosage of 1125 mg q8h in EFV-receiving patients.
Overall, 68% of the TVR receiving patients had a negative HCV viral load at week 4 (rapid virological response) compared to none with SOC. At follow-up week 12, 74% of the TVR receiving patients had a negative HCV viral load compared to 45% with SOC.
Seven patients experienced HCV viral breakthrough, defined as HCV RNA >100 IU/ml after HCV RNA undetectability or a 1 log10 increase from nadir. Two TVR patients had to stop treatment at week 4 and 8 because of stopping rules (HCV viral load >1000 IU/ml).
Side effects such as fatigue, nausea, pruritus and headache were more common in TVR treated patients. A mild to moderate rash occurred also more often, but did not lead to treatment discontinuation. Three patients stopped TVR treatment because of adverse events, one because of jaundice, one because of anaemia and one because of cholecystolithiasis (all in ATV arm). As trough levels (Cmin) and AUC of ATV both increase under TVR, increased total bilirubin levels can be expected under the co-administration of both drugs particularly as ribavirin induced haemolysis may further contribute to hyperbilirubinemia.
In study PO5411, 98 patients received a 4-week lead-in with pegylated interferon alpha-2b (Peg2b) and ribavirin [33]. Sixty-four patients received then 44 weeks of BOC/Peg2b/RBV compared to 34 patients with Peg2b/RBV alone. All patients had ART consisting of tenofovir/emtricitabine or lamivudine/abacavir plus a boosted HIV PI or maraviroc or RAL; NNRTIs were not allowed. BOC was given at the standard dose of 800 mg TID. A negative HCV RNA was seen in 42.2% of BOC-treated patients at week 8 (4 weeks after end of lead-in) compared to 14.7% in the SOC arm. At follow-up week 12, 61% of BOC-treated patients compared to 27% of SOC-treated patients had undetectable HCV RNA [32]. Three BOC-treated patients discontinued because of treatment failure. The most common side effects were neutropenia, dysgeusia, vomiting, pyrexia, headache and decreased appetite. Nine BOC-treated patients discontinued the study because of an adverse event. In both studies, there were no unexpected trends in CD4 counts or HIV RNA levels [33].
Management of HIV-HCV co-infected patients after DAA licensing
With the availability of TVR and BOC, a sophisticated decision making is crucial in HIV-HCV co-infected patients to increase cure rates and to avoid resistance development.
The decision whether to treat an HIV-HCV co-infected patient with DAAs or to defer treatment mainly depends on the stage of HIV disease, liver fibrosis stage and the rapidity of fibrosis progression, as these parameters define treatment necessity.
Patients with a CD4 count below 500/mm3 should benefit from an antiretroviral treatment first, because immunodeficiency is associated with lower SVR rates [34].
Liver biopsy is the gold-standard to estimate fibrosis stage. However, it is an imperfect tool, as the sampling of biopsy even with a centralized assessment with one experienced pathologist is major factor of variability [35]. Transient elastometry by Fibroscan® has proven its accuracy also in HIV-HCV co-infected patients [36]. Many serum biomarkers have also been validated in co-infected patients and especially Fibrotest®, Hepascore® and Fibrometer® are useful tools in this setting [37].
Preferably, two methods should be combined for an accurate result [38].
In newly diagnosed HCV, the first step will be to distinguish between acute and chronic disease. Acute HCV will be treated as before with a 24–48 weeks treatment course of pegylated interferon alpha (PegIFN) and ribavirin (RBV) [39]. If HCV infection is chronic, any HIV co-infected patient should possibly benefit from triple therapy to increase cure rates. If fibrosis estimation shows no or mild fibrosis (F0F1, Metavir), treatment can generally be deferred. If fibrosis estimation shows significant fibrosis (F2F3, Metavir) treatment should be inforced by an HCV PI. A ‘classic’ treatment with PegIFN and RBV may only be considered for HCV genotype 1 patients if the HCV viral load is below 800000 IU/ml, the favourable IL28B CC genotype (rs 12979860) is present, the CD4 count is high and in the absence of insulin resistance. If cirrhosis is present but not decompensated (Child-Pugh stage A), the patient should be treated with triple therapy (Fig. 1).
Figure 1. Management of newly diagnosed HIV-HCV coinfected genotype-1 patients.
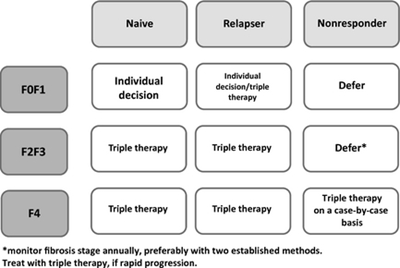
In patients who have already undergone a therapy with PegIFN and RBV but did not achieve sustained response, the decision should be made upon fibrosis stage and former treatment outcome (Fig. 2).
Figure 2. Management of HIV-HCV coinfected genotype-1 patients according to fibrosis stage and prior treatment outcome.
Former relapser should be treated with triple therapy if fibrosis is ≥F2 (Metavir) because the chances of cure are very high. If fibrosis is not present or mild (F0F1, Metavir) the decision should be made individually.
Former non-responders should not be treated with triple therapy because of high risk for failure [4] and resistance development if fibrosis is mild (F0F1, Metavir) or even significant (F2F3, Metavir). However, these patients need annually fibrosis estimation to observe a possible progression [39]. Patients with compensated cirrhosis (Child-Pugh stage A) should be treated with triple therapy.
Again, it appears important that prior to commencing HCV triple therapy HIV therapy needs to be optimized according to the available drug–drug interaction data: Zidovudine, stavudin and didanosin are furthermore not recommended in the setting of a ribavirin-containing regimen because of increased toxicity [41, 42]. Tenofovir/emtricitabine or lamivudine or abacavir/lamivudine remains the recommended nucleoside backbone.
Efavirenz appears to be a safe partner when TVR is used, but TVR doses have to be increased to 1125 mg TID. The choice of an HIV protease inhibitor is limited to boosted ATV when combined with TVR. Moreover, RAL has demonstrated favourable interaction profiles in healthy volunteers [25]. Recommendations for ART combinations with BOC are limited as only very few drug–drug-interaction studies have been performed [26, 27, 28], but preliminary data exist from the phase II trial. Raltegravir appears to be a safe option in this setting. Efavirenz substantially reduces BOC levels, whether this is clinically significant remains unclear until more data becomes available.
Conclusion
The approval of TVR and BOC will create new chances of cure also for HIV-HCV co-infected patients. Triple therapy will increase SVR rates in this setting, and drug–drug interactions appear to be manageable for the majority of patients. Nevertheless, a cautious approach might be preferable as new protease inhibitors with higher efficacy, lower pill burden, less pronounced interaction profiles and hopefully fewer side effects as well as new drug classes (also for non-genotype 1 patients) are on their way. Till then, a sophisticated management of drug–drug interactions will be crucial for a successful treatment.

No comments:
Post a Comment