Provided by NATAP
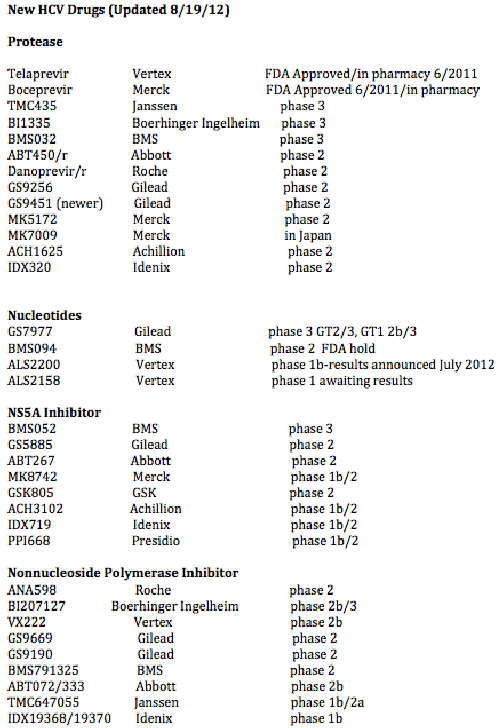
written for NATAP by Jules Levin: Currently approved are 2 protease inhibitors telaprevir & boceprevir. In phase 3 now are 4 drugs: 2 proteases TMC435 & BI1335; nucleotide GS7977; and NS5A BMS052. Also in phase 3 is Peg Lambda, a peginterferon that has showed in trials similar efficacy to current Peginterferons with little of the side effect. Phase 3 for these drugs should be finished in about one year with varying finish timelines between these drugs. Abbott is about to start phase 3 studies, they have 4 drugs in 3 classes: protease, NS5A, HCV polymerase nonnucleoside inhibitors (NNIs). Gilead has drugs in 4 classes: nucleotide, protease, HCV polymerase nonnucleoside inhibitors (NNIs), NS5A. BMS has drugs in 4 clases: NS5A, HCV polymerase nonnucleoside inhibitors (NNIs), nucleotide (trial just suspended, evaluating), protease; Roche/Genentech has drugs in 3 classes: protease, nuke, HCV polymerase nonnucleoside inhibitors (NNIs). Vertex has drugs in 3 classes: protease, nucleotide, HCV polymerase nonnucleoside inhibitors (NNIs). Merck has drugs in 2 classes: protease in development in addition to boceprevir, NS5A. Tibotec has protease TMC435 in phase 3 now with other drugs further back in development.
So in about 1 year we will have 2 brand new classes of drugs BMS052 the potent NS5A, GS7977 the potent nucleotide, plus the 2 new proteases currently in phase 3 BI1335 & TMC435. At this time we don't know if patients & clinicians will be able to combine the NS5A+GS7977 or a 3-drug combination of a protease TMC435 or BI1335 + the NS5A BMS052+GS7977. A small phase 2 study at EASl in April of about 40 patients showed SVR rates of 100% using GS7977+BMS5435, but there is not a followup study ongoing with these 2 drugs. There is an ongoing study now in null responders lookimg at TMC435+GS7977, we await these data, expecting good results. For null responders we have some data below in small studies finding that 2 BMS orals, the protease+NS5A + peg/RBV could cure 100% of patients.
Is there any doubt that we should be able to achieve 100% cure rates for all treatable patients?
Following this chart are links to the key studies recently reported that capture the data for these drugs.
Phase III Hallmark QUAD: ASV+DCV+Peg+Rib (Nulls/Partials) asunaprevir+BMS NS5A
Phase III Hallmark QUAD: ASV+DCV+Peg+Rib (Nulls/Partials)
This study is currently recruiting participants.
Verified July 2012 by Bristol-Myers Squibb
First Received on April 5, 2012. Last Updated on July 26, 2012
Phase III Hallmark DUAL: ASV+DCV (Nulls/Partials, Intolerants/Ineligibles. Naives)
Phase III Hallmark DUAL: ASV+DCV (Nulls/Partials, Intolerants/Ineligibles. Naives)
This study is currently recruiting participants.
Verified July 2012 by Bristol-Myers Squibb
First Received on April 18, 2012. Last Updated on July 23, 2012 EASL /AASLD Key Studies: interferon-free + studies for key new oral HCV drugs:
EASL: TMC435 with peginterferon and ribavirin in treatment-experienced HCV genotype 1 patients: the ASPIRE study, a randomised Phase IIb trial - (04/19/12)
EASL: TMC435 in patients infected with HCV genotype 1 who have failed previous pegylated interferon / ribavirin treatment: Virologic analyses of the ASPIRE trial - (04/19/12)
EASL: Once Daily GS-7977 Plus Ribavirin in HCV Genotypes 1-3: The ELECTRON Trial - (04/21/12)
EASL: GS-7977 + PEG/RBV in HCV Genotype 1: The ATOMIC Trial An End To Response-Guided Therapy - (04/20/12)
EASL: GS-7977 400 mg QD Safety and Tolerability in the Over 500 Patients Treated for at Least 12 Weeks - (04/25/12)
AASLD: SILEN-C3: treatment for 12 or 24 weeks with BI 201335 combined with peginterferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype-1 HCV infection - (11/07/11)
Once-daily NS5A Inhibitor (BMS-790052) Plus Peginterferon-alpha-2a ... Apr 17, 2010 ... Once-daily NS5A Inhibitor (BMS-790052) Plus Peginterferon-alpha-2a And Ribavirin Produces High Rates Of Extended Rapid Virologic Response In ... www.natap.org/2010/EASL/EASL_32.htm
BMS-790052 is a First-in-class Potent Hepatitis C Virus (HCV) NS5A .
------------------------------------
Key Recent Developments and other EASL Studies of note:
EASL: In Vitro Resistance Analysis of Merck's HCV NS5a Inhibitor MK-8742 Demonstrates Increased Potency AgainstClinical Resistance Variants and Improved Resistance Profile - (04/23/12)
EASL: Safety and Antiviral Activity of ABT-267, a Novel NS5A Inhibitor, During 3-Day Monotherapy: First Study in HCV Genotype-1 (GT1)-Infected Treatment-Naïve Subjects - (04/25/12)
EASL: Antiviral Activity and Resistance Profile of the Novel HCV NS5A Inhibitor GS-5885 - (04/25/12)
A Phase 2a Study of BMS-791325, an NS5B Polymerase Inhibitor ...
www.natap.org/2012/EASL/EASL_60.htm
A Phase 2a Study of BMS-791325, an NS5B Polymerase Inhibitor, With Peginterferon Alfa-2a and Ribavirin in Treatment-Naive Patients With Genotype
VX-222 Vertex HCV polymerase nonnucleoside inhibitors (NNIs)Polymerase Inhibitor 3 Days Monotherapy
www.natap.org/2010/EASL/EASL_02.htm
VX-222 Vertex HCV polymerase nonnucleoside inhibitors (NNIs)Polymerase Inhibitor 3 Days Monotherapy. Reported by Jules Levin EASL Apr 14-18 2010. Vienna Austri
Vertex QUAD Therapy Yielded 83-93% SVR with 12 weeks duration ...
www.natap.org/2012/APASL/APASL_11.htm
VX-222 is a selective, noncompetitive inhibitor of the hepatitis C virus (HCV) NS5B polymerase.1 Telaprevir (TV
BMS Suspends Study of Nucleotide BMS094 Formerly INX189 - (08/02/12)
Vertex Nucleotide Analysis - (08/01/1
Gilead Begins Single Pill Hepatitis C Study for 2014 Approval - (07/27/1
Medivir announces an interferon-free phase II combination trial with TMC435 and daclatasvir to commence shortly - (07/10/12)
Open-Label Study of GS-7977 + Ribavirin Pre-Transplant - (06/22/1
Idenix Announces Positive Clinical Data for HCV Drug Candidates IDX184 and IDX719(NS5A) - (06/20/12
EASL: Use of Telaprevir Plus Peg Interferon/Ribavirin for Null Responders Post OLT With Advanced Fibrosis/Cholestatic Hepatitis C - (05/04/12)
EASL: NOVEL NS5A INHIBITOR ACH-2928 PHASE 1 RESULTS IN HEALTHY VOLUNTEERS AND HCV GT-1 PATIENTS - (05/02/12)
EASL: CUPIC: French Early Access Program - Compassionate Use of Protease Inhibitors in Viral C Cirrhosis - (04/26/12)
EASL/2011: Genotypic and Phenotypic Characterization of NS3 Variants Selected in HCV-Infected Patients Treated with ABT-450 - (04/05/11)
EASL: Presidio Pharmaceuticals Announces Phase 1a-1b Clinical Results with PPI-668, a Potent Pan-genotypic HCV NS5A Inhibitor - (04/25/12
ANA-598 HCV Non-Nuke Phase 2b Study Preliminary Data Results Background to Phase IIb Study - (10/14/11)
New Data Reported on INX-189, HCV Nucleotide
www.natap.org/2011/HCV/112911_01.htm
Nov 29, 2011 - top-line safety and antiviral data from its ongoing clinical trial designed to evaluate additional doses of INX-189, an oral nucleotide polymerase
No comments:
Post a Comment