Provided by NATAP
this study reports new HCV therapy can in the near future reduce cirrhosis & death by 50-280% in Western Europe (see graphs)....."this study also underlines the urgent need for reinforcement of HCV screening and access to therapy."
Gastroenterology October 2012
SYLVIE DEUFFIC-BURBAN,*, PIERRE DELTENRE,, MARIA BUTI, TOMMASO STROFFOLINI,# JULIE PARKES,** NIKOLAI MUHLBERGER, UWE SIEBERT,,, CHRISTOPHE MORENO, ANGELOS HATZAKIS,## WILLIAM ROSENBERG,*** STEFAN ZEUZEM, and PHILIPPE MATHURIN,
"In conclusion, the present study clearly shows the benefit of antiviral therapy in European countries, amplified by the use of PIs. The present data should help public health authorities to optimize the impact of such therapy on morbidity and mortality."
"the total number of patients with cirrhosis (including complications) is expected to peak in 2020 for Belgium, 2021 for France, 2023 for Germany, 2030 for Spain, and 2033 for the United Kingdom in the absence of treatment; for Italy, the peak was reached in 2008. These differences in fibrosis stage distribution over time are related to patterns of HCV infection. Belgium, France, and Germany present patterns similar to that predicted by Davis et al in the United States."
"The future expected regimen (assumed to be available in 2017) would considerably impact the overall HCV-related incidence of cirrhosis and death from 2017 to 2026, with an even greater effect with reinforcement of HCV screening and treatment access (Figure 4A and B). Detailed results are shown in Supplementary Table 7."
"From 2002 to 2011, antiviral therapy reduced the cumulative incidence of cirrhosis by 7.1% and deaths by 3.4% overall. Reductions in incidence and mortality values ranged from 4.0% and 1.9%, respectively, in Italy to 16.3% and 9.0%, respectively, in France. From 2012 to 2021, antiviral treatment of patients with HCV genotype 1 infection that includes protease inhibitor-based triple therapy will reduce the cumulative incidence of cirrhosis by 17.7% and mortality by 9.7% overall. The smallest reduction is predicted for Italy (incidence reduced by 10.1% and mortality by 5.4%) and the highest is for France (reductions of 34.3% and 20.7%, respectively)."
Figure 3 from Jules of NATAP: as you can see in Figure 3 improved access to treatment reduces cirrhosis & death 50-280%, and this analyses appear to only consider triple therapy with a PI+ Peg/Rbv, not even all oral 100% cure rates with 3-4 orals IFN-free therapy.
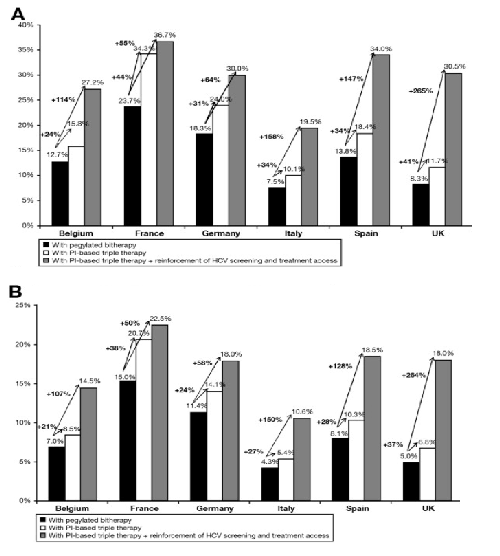
Treatment impact from 2012 to 2021 for G1: reduction in cumulative incidence of HCV-related (A) cirrhosis and (B) death for each country, considering pegylated bitherapy, PI-based triple therapy alone, or PI-based triple therapy and reinforcement of HCV screening and treatment access.
(Click on picture to enlarge)
Background & Aims
The dynamics of hepatitis C virus (HCV) infection, as well as screening practices and access to therapy, vary among European countries. It is important to determine the magnitude of the effects of such differences on incidence and mortality of infection. We compared the dynamics of infection and screening and treatment practices among Belgium, France, Germany, Italy, Spain, and the United Kingdom. We also assessed the effects of treatment with pegylated interferon and additional effects of triple therapy with protease inhibitors.
Methods
We created a country-specific Markov model of HCV progression based on published epidemiologic data (on HCV prevalence, screening, genotype, alcohol consumption among patients, and treatments) and reports of competitive and hepatocellular carcinoma mortality for the 6 countries. The model was used to predict the incidence of HCV-related cirrhosis and its mortality until 2021 for each country.
Results
From 2002 to 2011, antiviral therapy reduced the cumulative incidence of cirrhosis by 7.1% and deaths by 3.4% overall. Reductions in incidence and mortality values ranged from 4.0% and 1.9%, respectively, in Italy to 16.3% and 9.0%, respectively, in France. From 2012 to 2021, antiviral treatment of patients with HCV genotype 1 infection that includes protease inhibitor-based triple therapy will reduce the cumulative incidence of cirrhosis by 17.7% and mortality by 9.7% overall. The smallest reduction is predicted for Italy (incidence reduced by 10.1% and mortality by 5.4%) and the highest is for France (reductions of 34.3% and 20.7%, respectively).
Conclusions
Although HCV infection is treated with the same therapies in different countries, the effects of the therapies on morbidity and mortality vary significantly. In addition to common guidelines that are based on virologic response-guided therapy, there is a need for public health policies based on population-guided therapy.
Knowledge of the natural history of hepatitis C virus (HCV) infection can help to develop models for predicting the future course of HCV infection.1, 2, 3, 4, 5, 6, 7 Accuracy of a predictive model of spread of HCV infection requires elucidation of the dynamics of infection, the epidemiologic pattern of contamination routes, and distribution of genotypes. These parameters vary between countries and must be evaluated by robust studies at a national level.
The impact on disease progression of HCV eradication via current antiviral therapy with pegylated interferon (PEG-IFN) and ribavirin (RBV) is well known.8, 9 Future therapeutic combinations using triple therapy of directly acting antivirals, namely protease inhibitors (PIs), with PEG-IFN and RBV should form the basis of treatment of naive10, 11, 12, 13, 14, 15 and experienced16, 17, 18 with HCV genotype 1 (G1) infection. However, no clinical studies have assessed the impact of antiviral therapy on long-term morbidity and mortality because it is unethical to maintain patients without therapy. Only a modeling approach can address this issue and predict its impact on a population.5
The development of country-specific models enables comparison of HCV natural history, prevalence according to fibrosis stage, and impact of therapy on HCV burden across countries. Recent studies pointed out substantial discrepancies in Europe in terms of HCV burden19 and access to antiviral therapy.20 Indeed, the prevalence of HCV ranged from 0.6% in Germany to 4% in Italy, and the number of patients treated ranged from 16% of HCV prevalent cases in France to 3% in Italy and the United Kingdom.19, 20 Until now, the impact on HCV morbidity and mortality of differences in access to treatment in different European countries has been ignored. A modeling approach taking into account national characteristics such as epidemiologic patterns, natural history, screening, and treatment rates may help to adapt therapeutic strategies and public health policies to the national HCV burden and "population-guided therapy."
In this study, we built country-specific models for Belgium, France, Germany, Italy, Spain, and the United Kingdom. Our main objectives were to (1) compare these countries in terms of dynamics of infection, natural history, screening, and treatment practices; (2) assess, by country, the impact of pegylated bitherapy; and (3) predict the additional impact of triple therapy with PIs.
Discussion
The dynamics of infection, natural history, screening, and therapeutic access are important components affecting progress in antiviral therapy in terms of morbidity and mortality. Taking into account these parameters, we show here that (1) the dynamics and natural history of HCV infection differ drastically in Belgium, France, Germany, Italy, Spain, and the United Kingdom; (2) the impact of antiviral therapy in reducing the incidence of cirrhosis and deaths substantially varies across these countries; (3) for G1 patients with HCV, the impact of PI-based triple therapy from 2012 to 2021 might vary from 10.1% to 34.3% for HCV-related cirrhosis and from 5.4% to 20.7% for HCV-related mortality; and (4) ideally, 75% of HCV screening by 2015 and one patient out of two treated in 2015 (with triple therapy for G1-infected patients and pegylated bitherapy for other genotypes) in all these countries would impact HCV morbidity and mortality from 2012 to 2021 (from 19.5% to 36.7% and 10.6% to 22.5%, respectively). The present study provides a new concept that could help in creating public health policies for population-guided therapy.
The present modeling study showed significant differences in the spread of HCV infection, leading to specific patterns of natural history in these European countries. Indeed, the total number of patients with cirrhosis (including complications) is expected to peak in 2020 for Belgium, 2021 for France, 2023 for Germany, 2030 for Spain, and 2033 for the United Kingdom in the absence of treatment; for Italy, the peak was reached in 2008. These differences in fibrosis stage distribution over time are related to patterns of HCV infection. Belgium, France, and Germany present patterns similar to that predicted by Davis et al in the United States.4 In Italy, an intensive epidemic wave appeared to have occurred during the 1950s and 1960s, mainly associated with poor hygiene during invasive procedures (eg, surgery, gynecology, dentistry, vaccinations, injection of antibiotics and vitamins), whereas in other countries the most intensive epidemic waves occurred during the 1980s, mainly related to transfusions and intravenous drug use. Consequently, in Italy, although antiviral therapy should reduce HCV morbidity and mortality, it will not affect the year or magnitude of the peak; in all other countries, antiviral therapy will have an impact on both (results not shown).
Our main concern was to develop country-specific models that fit "real-life" data integrating the probability of being treated based on clinicians' decisions and health policies. We used PEG-IFN sales obtained from GERS (for France) and IMS (for other countries). These data enabled us to estimate the annual likelihood of being treated among screened patients, and each country-specific model was calibrated according to the number of treated patients. These numbers are a consequence of the contraindications and side effects profile of the IFN-based regimen. Indeed, clinicians aware of the side effects profile of an IFN-based regimen adapt their decisions according to patient characteristics. For example, few patients aged 70 years in F0-F1 were treated in 2011 (from 0.8% in Italy to 4.0% in France; data not shown). Overall, estimated likelihoods depend on country, year, genotype, treatment history (naive vs re-treated patients), fibrosis (≥F2 vs F0-F1), and the presence of alcohol abuse. In addition, the proportion of prior nonresponders with F3-F4 in our model was based on clinical practice, which differs from licensing trials. Indeed, the proportion of F3-F4 among treated patients depended on the distribution of fibrosis among screened patients, which was the result of natural history modeling, the higher likelihood of treatment among F2-F4 patients, and the fact that naive F3-F4 patients had a lower probability of SVR and therefore higher probability of being nonresponders. The latter 2 points (higher likelihood of being treated and lower SVR probability) synergistically increment the proportion of prior nonresponders with F3-F4.
During the past decade (2002-2011), the overall reduction in morbidity and mortality was much higher in France (-16.3% and -9.0%, respectively) compared with other countries (ranging from -4.0% to -11.2% and -1.9% to -5.5%, respectively). Although the use of PIs in G1 patients will increase the impact of antiviral therapy on morbidity and mortality during the next decade (2012-2021), this study also underlines the urgent need for reinforcement of HCV screening and access to therapy. Indeed, the hypothetical scenario involving use of PIs with the same targeted level of HCV screening and access to therapy for all countries indicates that all countries except Italy will catch up with France. Moreover, sensitivity analysis assuming progress expected due to drugs currently in development clearly confirmed that any progress in SVR will mainly have an impact on HCV-related morbidity and mortality if accompanied by an increase in HCV screening and access to therapy. These data reveal the complex role of the spread and natural history of HCV infection and emphasize the need for innovative public health strategies in population-guided therapy. This approach of targeted public health strategies may also be useful for countries outside of Europe but will necessitate data for country-specific models.
Until now, experts have been focusing primarily on management of patients according to virologic kinetics and disease severity. The present study shows that, although clinicians from different countries use the same drugs according to therapeutic guidelines provided by scientific organizations, the impact on morbidity and mortality across countries varies as a result of differences in the spread of HCV infection, screening, and access to therapy. Therapeutic guidelines are first set up for patient care at an individual level but do not have the tools for measuring treatment impact on morbidity and mortality at a population level. This study suggests that delaying treatment in patients with F0 or F1 until they progress to F2 is efficient. However, this strategy is difficult in clinical practice because of the need for an efficient diagnostic method to detect fibrosis progression from F0-F1 to F2 as well as optimization of the interval and frequency of diagnostic testing specifically adapted to patient characteristics. Our results should be useful to national experts when proposing therapeutic guidelines.
As expected in a modeling approach, the present work had limitations associated with data and assumptions. Country-specific models were developed after identifying at least one expert in each country so as to obtain the most accurate analyses. Moreover, the country-specific data used to adjust or calibrate each model led to constraints that made certain assumptions impossible. However, one limitation concerns the assumption of the past incidence of HCV infections. We assumed that the past incidence of HCV infections decreased from 1990 at the same proportions as those observed in the United States, except for Italy and the United Kingdom. Indeed, the same assumption was first made for the United Kingdom, but it did not enable a good fit for reported HCC deaths related to HCV. This assumption seemed valid for countries in which transfusion-induced infections were predominant before 1990, but not for the United Kingdom, with infections mainly occurring in intravenous drug users. Moreover, in an earlier work performed in France, the impact on future morbidity and mortality of the variation in the decrease of the incidence of HCV after 1990 was found to be small.5 Secondly, we used data on PEG-IFN purchases in each country during 2002-2005 to calibrate our model. Limitations exist concerning our assumptions and parameters used to convert sales into patient figures. However, our strategy consisted of identifying experts possessing both PEG-IFN sales data and the expertise needed to convert these sales into numbers of treated patients.20 Thirdly, liver transplantation was not considered. Indeed, liver transplantation for HCV-related liver failure or HCC represents only a small number in comparison to HCV mortality. For example, in Spain, the country with the highest liver donation rate in Europe, 378 liver transplantations for HCV-related liver failure and HCC were performed in 2009, but there were 4500 HCV-related deaths. In addition, liver transplantation does not eradicate the disease, because patients are still at risk for disease progression and 5-year survival is approximately 65%.54, 55 Thus, liver transplantation should not significantly affect results.
In conclusion, the present study clearly shows the benefit of antiviral therapy in European countries, amplified by the use of PIs. The present data should help public health authorities to optimize the impact of such therapy on morbidity and mortality.
Results
HCV Natural History
Figure 1 shows the distribution of fibrosis stage over time using the no-treatment scenario for each country. Belgium, France, and Germany showed the same pattern of HCV natural history across fibrosis stages. For those countries, cases of cirrhosis and its complications, such as decompensated cirrhosis and HCC (F4 and its complications), would have stabilized around 2020-2024. In Spain and the United Kingdom, cases of cirrhosis and complications from the disease should continue to increase over the study period, reaching a peak in 2030 and 2033, respectively (not shown). In contrast, in Italy, due to a less recent HCV epidemic, cases of cirrhosis and complications would have already reached their peak of prevalence (2008 in the absence of treatment).
Impact of HCV Treatment on HCV-Related Cirrhosis and Mortality
Impact of pegylated bitherapy from 2002 to 2011
Table 1 shows the impact of treatment over the past 10 years (2002-2011), giving estimates of cumulative HCV-related cirrhosis and HCV-related deaths without and with treatment. Overall, HCV treatment reduced the cumulative incidence of cirrhosis from 414,400 (95% CI, 393,100-432,500) to 385,000 (95% CI, 365,600-401,200) (ie, -7.1%) during this period. The impact of treatment on reduction in HCV-related cirrhosis is shown in Figure 2A for each country; reduction varied from 4.0% in Italy to 16.3% in France. As expected, this reduction was higher for G2/3 (from 138,400 to 122,300 cases of cirrhosis; -11.6%) than for G1/4 (from 276,000 to 262,800 cases of cirrhosis; -4.8%). This difference was found for all countries (Figure 2A).
Similarly, HCV treatment reduced the cumulative incidence of deaths from 286,000 (95% CI, 273,400-298,000) to 276,400 (95% CI, 264,300-287,800) (ie, -3.4%) from 2002 to 2011. The weakest impact was obtained for Italy (-1.9%) and the strongest for France (-9.0%) (Figure 2B). Again, HCV treatment had a greater impact on G2/3 (from 93,000 to 87,400 deaths; -6.0%) than on G1/4 (from 193,000 to 188,900; -2.1%), and this difference was found for all countries (Figure 2B).
Future Impact of Antiviral Therapy Integrating Triple Therapy on G1 From 2012 to 2021
Overall impact on patients with HCV
Table 2 shows the impact of treatment over the next 10 years (2012-2021), with the cumulative incidence of HCV-related cirrhosis and HCV-related deaths predicted without and with treatment, including PI-based triple therapy for G1 (most conservative assumption, assuming no change in the evolution of screening rates or treatment practices for each country). Overall, HCV treatment would reduce the cumulative incidence of cirrhosis from 400,300 (95% CI, 378,300-412,600) to 318,100 (95% CI, 301,400-328,700) (ie, -20.5%) from 2012 to 2021 and the cumulative incidence of deaths from 316,200 (95% CI, 300,600-330,000) to 277,600 (95% CI, 264,200-289,200) (ie, -12.2%) from 2012 to 2021. Again, the lowest impact would be observed in Italy (12.9% and 7.5%, respectively) and the highest in France (38.9% and 25.5%, respectively) (not shown).
Specific Impact on G1 Patients With HCV
PI-based triple therapy would considerably impact the HCV-related incidence of cirrhosis in G1 patients, with relative impact varying from 10.1% (Italy) to 34.3% (France) and intermediate relative impacts of 11.7% in the United Kingdom, 15.8% in Belgium, 18.4% in Spain, and 24.0% in Germany (Figure 3A). The additional impact of PI-based triple therapy versus pegylated bitherapy would vary between 24% in Belgium and 44% in France (Figure 3A). Similarly, PI-based triple therapy would affect the HCV-related incidence of deaths for G1, with relative impact varying from 5.4% in Italy to 20.7% in France (Figure 3B). The additional impact of PI-based triple therapy versus pegylated bitherapy would vary from 21% in Belgium to 38% in France (Figure 3B).
If we now consider that the availability of triple therapy will be accompanied by reinforced screening and treatment access (less conservative assumption, assuming that 75% of HCV-infected patients will be screened by 2015 and one G1-infected patient out of two will be treated in 2015 with PI-based triple therapy), the overall relative impact of PI-based triple therapy on the HCV-related incidence of cirrhosis would be 27.4% (from 19.5% in Italy to 36.7% in France) (Figure 3A). Moreover, the additional impact of PI-based triple therapy versus pegylated bitherapy would be dramatically increased. Similarly, in the less conservative scenario, the relative overall impact of PI-based triple therapy on the HCV-related incidence of death would reach 15.0% (from 10.6% in Italy to 22.5% in France) (Figure 3B), and the additional impact of PI-based triple therapy versus pegylated would be dramatically increased.
Sensitivity Analyses
The scenario that consists of withholding treatment until reaching F2 is more efficient than the others ("Never treating patients with F0 or F1" or "Not treating patients with F0 or F1 until they reach F3"), as shown in Supplementary Table 6.
The future expected regimen (assumed to be available in 2017) would considerably impact the overall HCV-related incidence of cirrhosis and death from 2017 to 2026, with an even greater effect with reinforcement of HCV screening and treatment access (Figure 4A and B). Detailed results are shown in Supplementary Table 7.
Materials and Methods
Overview
We used a country-specific back-calculation method and a state-transition Markov model to first reconstruct the past incidence of HCV infection and simulate progression of HCV disease among newly HCV-infected cohorts in Belgium, France, Germany, Italy, Spain, and the United Kingdom. Next, we projected country-specific HCV-related morbidity and mortality and assessed the impact of therapy for each country.
Natural History of Disease in Newly HCV-Infected Cohorts
Newly HCV-infected cohorts were derived from country-specific past incidence of HCV infection, with the latter estimated during a back-calculation process. Past incidence of HCV infection was assumed to follow a logistic function until a peak of infection in 1989 for all countries except Italy. For Italy, where a more intense epidemic wave occurred during the 1950s to 1960s via iatrogenic transmission due to use of unsterilized material,21 the peak of infection was set at 1969. Past incidence of HCV infection was then assumed to decrease in the same proportions as those observed in the United States thereafter (94% decrease between 1989 and 2008),22 except for Italy and the United Kingdom. For Italy, we used the same decrease as that estimated by Mariano et al,21 that is, from a 21% decline in 1970 to a 98% decline in 2000. For the United Kingdom, where most HCV infections occurred in intravenous drug users, the decrease was assumed to be lower than in the United States,23 that is, a 46% decrease between 1989 and 1998 followed by stabilization.
Newly HCV-infected cohorts in each country were characterized by age at the time of HCV infection, sex, genotype (see Supplementary Table 1), and alcohol abuse status (T. Stroffolini, personnal communication, 2010, for Italian data).24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 The Markov model (see Supplementary Materials and Methods) used to simulate progression of these newly HCV-infected cohorts is detailed elsewhere.5 Death rates from causes other than HCV (competitive mortality) were assumed to be all-cause mortality from country-specific life tables.
Impact of HCV Treatment on Disease Progression
As previously determined, antiviral treatment effects were incorporated by estimating likelihood of being screened for HCV, of being treated, and of reaching sustained virologic response (SVR) after treatment according to the HCV genotype for naive and previously treated patients.
The likelihood of being screened for HCV differed between countries (see Supplementary Table 1). France was the only country for which we had 2 estimates of HCV screening: 24% in 1994 and 57% in 2004.38 As previously determined,5 we assumed a linear increase in HCV screening between 1994 and 2004 but assumed a lower increase in HCV screening thereafter so as to be at a 62% level in 2009. In Belgium, a study based on recall of patients after use of inactive batches of Cidex (Johnson & Johnson Medical, UK) disinfection solution in Belgian hospitals in 2000 estimated that 99 of the 265 (37%) positive patients already knew their status.39 For Germany, based on the work of Zehnter et al,40 we estimated that 40% of HCV-infected persons were screened in Germany in 2004, similar to data from a European report41 (see Supplementary Materials and Methods for details). In Italy, Mariano et al estimated that, among HCV RNA-positive individuals detected on screening, 40% already knew of their infection in 2005.21 For Spain, we extracted an HCV screening rate of 33% compatible with the estimate that 53% of HCV-related hepatocellular carcinomas (HCCs) were screened during 2008-2009 according to Varela et al42 (see Supplementary Materials and Methods for details). For the United Kingdom, we assumed an HCV screening rate of 30% in 2004 according to estimates of diagnosed populations found in national reports.43, 44 For those 5 countries, we assumed a linear increase in HCV screening starting at approximately 3% at the beginning of treatment (1991).
The likelihood of being treated for patients aware of their infection also differed between countries and was fitted to the 2002-2005 number of treated patients extracted from PEG-IFN sales obtained from GERS (http://www.gie-gers.fr/) for France and from the IMS (http://www.imshealth.com/portal/site/imshealth) for other countries (see Supplementary Materials and Methods).
The likelihood of attaining an SVR after treatment was obtained from the literature.8, 9, 10, 11, 13, 15, 17, 45, 46, 47
Procedure
During the process, the model was first fitted to reported age-specific annual HCC deaths related to HCV (see Supplementary Materials and Methods) in each country and calibrated to country-specific HCV prevalence and PEG-IFN sales in 2002-2005 (see Supplementary Table 1, Supplementary Table 2).
Next, the model simulated HCV progression until 2021 for all HCV infections occurring until 2010, assuming that current treatment practices with pegylated bitherapy would be continued until 2021. The model also simulated HCV progression until 2021 in the absence of treatment for each country. We then assessed the availability of triple therapy with PIs starting in 2012 and leading to a higher SVR for G1-infected patients, which was 78% in F0-F2 and 62% in F3-F4 naive patients and 66% in F0-F2 and 48% in F3-F4 non-naive patients after correction for proportions of relapsers and nonresponders from the IDEAL study.11, 18, 48 The 2 treatment regimens (pegylated bitherapy and triple therapy for G1-infected patients) were compared with absence of treatment.
The relative impact of treatment (either pegylated bitherapy or triple therapy) was calculated by subtracting the cumulative incidence (either HCV-related cirrhosis or HCV-related deaths) estimated with treatment from that estimated without treatment, divided by the estimated incidence without treatment. Because it is important that clinicians be able to assess the impact of triple therapy compared with pegylated bitherapy, the additional impact of triple therapy compared with pegylated bitherapy was estimated by subtracting the relative impact of triple therapy from that of pegylated bitherapy, divided by the relative impact of pegylated bitherapy. To assess the impact of triple therapy, we first held the assumption that HCV screening rates and treatment access would remain unchanged (most conservative assumption). In an alternative scenario, we assumed that HCV screening rates and treatment access would increase for each country with the availability of triple therapy, leading to 75% of HCV-screened patients by 2015 and one patient out of two treated in 2015 with new triple therapy for G1 and pegylated therapy for other genotypes (less conservative assumption).
The 95% confidence intervals (CIs) were calculated from the estimated variance-covariance matrix of the estimated parameters. We redid the entire country-specific analyses from these lower and upper bounds to obtain a 95% CI of HCV-related morbidity and mortality.
Sensitivity Analyses
Controversies persist as to whether patients in F0 or F1 need to be treated. The group of G2/3 patients with the highest SVR is best suited for testing a strategy of withholding patients in F0 or F1. Evaluation of such a strategy requires testing 3 potential treatment scenarios with PEG-IFN and RBV: (1) never treating patients with F0 or F1, although some of them will progress; (2) not treating patients with F0 or F1 until they reach fibrosis F2; and (3) not treating patients with F0 or F1 until they reach fibrosis F3.
Given the rapid pace of HCV drug development, we integrated future regimens into the 10-year horizon (2012-2021), considering the first 5 years with dual therapy of PEG-IFN and RBV (non-G1) or triple therapy (G1) and the next 5 years with an IFN-free regimen for G2/3 treatment-naive patients and triple or quadruple therapy for other patients. However, a realistic evaluation of the future regimen requires at least a 10-year period following the year of start of this therapy, leading us to provide results for 2017-2026. We based our assumptions on results of clinical studies evaluating future treatment regimens that are nearing phase 3 testing and will probably be available in the near future.49, 50, 51, 52, 53 To remain conservative, and because most studies recruited patients without extensive fibrosis, we applied a 20% reduction in efficacy for F3-F4 (see Supplementary Table 5). We first held the assumption that the HCV screening rate and treatment access would remain unchanged. To underline the urgent need for combining progress in the SVR rate with reinforcement of HCV screening and access to therapy, we performed the same scenario assuming that 75% of HCV-infected patients will be screened by 2017 and one infected patient out of two will be treated in 2017 with the future treatment regimen.
Additional sensitivity analyses were performed and can be found in the Supplementary Materials and Methods.
No comments:
Post a Comment