From Journal of Viral Hepatitis
A Multicentric Prospective Study
E. Fransvea; P. Trerotoli; R. Sacco; V. Bernabucci; M. Milella; N. Napoli; A. Mazzocca; E. Renna; M. Quaranta; G. Angarano; E. Villa; S. Antonaci; G. Giannelli
Posted: 10/05/2012; J Viral Hepat. 2012;19(10):704-710. © 2012 Blackwell Publishing
Abstract and Introduction
Abstract
The combination of pegylated interferon (Peg-IFN) and ribavirin is currently the gold standard therapy in patients with HCV chronic infection. The duration of therapy, as well as the therapeutic dosage, depend on the genotype. Identification of the genotype and rapid virological response (RVR) are widely accepted as the most important predictors of clinical outcome during antiviral therapy but to optimize cost-benefits and to reduce possible side effects, further prognostic factors are needed. Squamous cell carcinoma antigens immunocomplex (SCCA-IC) has been reported to be increased in the serum of patients with liver cancer. In this multicentric prospective study, we investigated the serum levels of SCCA-IC in 103 patients with HCV chronic infection. Serum HCV-RNA was detected before the beginning of treatment, after 4, 12, 24 or 48 weeks, and at week 24 during follow-up. RVR, early virological response and sustained virological response (SVR) were assessed following the international guidelines. SCCA-IC levels were higher in responders (238 AU, interquartile difference 130–556 AU) and decreased significantly to 125 AU (70–290 AU). The mean baseline value in nonresponders was 149 AU (86.5–306.5 AU), but after 4 weeks of treatment the serum levels decreased to 115 AU (80–280 AU): the profile of reduction was different between patients with or without a positive SVR. Logistic regression with SVR as dependent variable identified as significant independent variables: the reduction in SCCA-IC after 1 month (OR = 4.82; 95% CI 1.39–16.67; P = 0.131) and a genotype other than 1 (OR = 0.094; 95% CI 0.21–0.42; P = 0.002); sex and age were also significant factors influencing SVR. SCCA-IC seems to be a reliable independent prognostic marker of therapeutic effectiveness in anti-HCV positive patients undergoing antiviral therapy.
Introduction
The gold standard treatment for chronic hepatitis C, to prevent or delay progression to liver cirrhosis and hepatocellular carcinoma (HCC), is currently the combination of pegylated interferon-α (Peg-IFN-α) with ribavirin.[1,2] The HCV genotype and a rapid virological response (RVR) have been widely recognized as the two most important prognostic factors for the response to antiviral therapy.[3,4] The international guidelines for the treatment of HCV patients have suggested that these prognostic factors may be usefully applied to tailor the therapeutic regimen and optimize the cost-benefit ratio.[5] Nevertheless, additional prognostic factors are urgently needed in clinical practice.
Recently, in a preliminary study, it was reported that serum levels of squamous cell carcinoma antigen immuno-complex (SCCA-IC) were decreased in patients with HCV-related cirrhosis who responded to antiviral therapy.[6] However, the limited number of patients so far investigated, and the lack of studies in patients with chronic HCV-related hepatitis do not allow definite conclusions to be drawn as regards the validity of this test in patients undergoing antiviral therapy. In particular, there are no data available correlating the use of SCCA-IC with RVR, widely recognized as the gold standard marker of clinical response to antiviral therapy.
SCCA-IC is an immuno-complex whereby IgM immunoglobulins link the serin inhibitor protease SCCA.[7,8] SCCA was first reported to be expressed in the liver of HCC patients and for this reason, was proposed as a potential biomarker for the detection of HCC.[9] SCCA-IC serum levels have been demonstrated to show a better diagnostic capacity than SCCA and have therefore been proposed for use in combination with alpha-fetoprotein as an additional biomarker for HCC detection.[10]
Aim of this study was to test the utility of SCCA-IC as a marker of response in patients with HCV chronic infection undergoing antiviral therapy. A multicentric prospective study was designed, in which serum samples were collected at baseline before starting the therapy, at RVR, early virological response (EVR) and 6 months after the end of therapy, and a cohort of patients with HCV chronic infection undergoing antiviral therapy were enrolled.
Materials and Methods
Patients
During the period 2007–2010, 103 patients referred to the following centres: Unit of Internal Medicine 'C. Frugoni' and of Infectious Diseases, University of Bari; Unit of Gastroenterology, University of Pisa; and Unit of Gastroentorology, University of Modena And Reggio Emilia were enrolled in this study.
Patients with liver cirrhosis, haematological abnormalities (haemoglobin level <12 g/dL in women and <13 g/dL in men; neutrophil count <1.5 × 103 cells/mL; platelet count <90 × 103 cells/mL), pre-existing severe psychiatric conditions (especially depression), severe cardiac disease, haemoglobinopathies, haemophilia, autoimmune diseases, human immunodeficiency virus (HIV) co-infection, previous liver transplantation and other causes of liver disease (hepatitis B virus infection, alcohol dependence) were excluded. Women unable or unwilling to practise contraception were also excluded.
Study Design
In total, 103 patients were recruited, 61 men and 42 women. Mean age was 53.4 year (±12.7 year) in men and 58 year (±9.5 year) in women, with a significant difference between the two groups (t-test = 2.05; P = 0.04).
Blood samples were collected at baseline before the beginning of therapy, after 4, 12, 24 and 48 weeks during treatment and at 24 weeks of follow-up. The study was not supported by any pharmaceutical company. Informed consent was obtained from all patients.
Patients were treated with weekly subcutaneous pegylated interferon (Peg-IFN)-α2a (180 μg/week) or Peg-IFN-α2b (1.5 μg/kg/week) plus oral ribavirin at a dosage of 800–1200 mg/day depending on pretreatment body weight (800 mg/day for weight <60 kg, 1000 mg/day for weight ≥ kg and <75 kg; and 1200 mg/day for weight ≥75 kg). This study was not randomized, and the physicians in charge of the patients chose the Peg-IFN at their own discretion. Patients with genotype 2 and 3 were treated for 24 weeks, and those with genotype 1 and 4 for 48 weeks. Virological response was evaluated at weeks 4, 12, 24 and 48 during treatment and at 24 weeks of follow-up by qualitative PCR (Amplicor; Roche Diagnostic System, France), with a sensitivity of 50 UI/mL. HCV genotype was determined before treatment in all patients with the INNO-LiPA HCV II kit (Bayer Diagnostics, Emeryville, CA, USA). Sustained virological response (SVR) was defined as the absence of detectable HCV-RNA in serum by qualitative PCR at the end of therapy and at week 24 of follow-up. Early virological response was defined as undetectable serum HCV-RNA or a reduction in HCV-RNA levels by at least 2 logs from baseline values at week 12 of treatment. Rapid virological response, was defined as undetectable serum HCV-RNA at week 4 of treatment. Patients with measurable HCV-RNA by qualitative PCR at the end of the follow-up period were considered nonresponders.
SCCA-IC Measurement
SCCA-IC serum concentrations were determined in sera previously collected and stored at −20 °C until use. Serum concentrations of SCCA-IC were measured as in our previous works, using ELISA commercial kits purchased from Xeptagen (Padua, Italy).
Statistical Analysis
Categorical variables are summarized as count and percentage. Chi-squared test was used to evaluate differences between independent groups. Comparisons were made by parametric tests if normally (Gaussian) distributed, nonparametric tests otherwise. Quantitative variables are summarized as mean and standard deviation. Differences of SCCA-IC, lacking a Gaussian distribution, are summarized as median and interquartile range and comparison was performed by Kruskal–Wallis for comparing multiple independent groups or Friedman test for nonparametric repeated measure analysis of variance. Multiple comparisons between paired or independent groups were performed using Wilcoxon test and P-values were adjusted taking into account the number of comparisons according Bonferroni.
The percentage variation, determined as the ratio between the difference of SCCA-IC at time t0 less SCCA-IC at time t-1, divided by SCCA-IC at time t-1, was calculated to evaluate the reduction in SCCA-IC concentrations between two consecutive time points. The percentage variation was then classified in two classes according to the median value. A logistic regression model was built to evaluate the effect on SVR of the class of SCCA-IC reduction (more than 12% after the first month and after the third month, more than 7% at the last follow-up visit), genotype (1 vs other), adjusted for age (classified as <58 vs≥58 year) and sex (M vs F).
To evaluate the concordance of percentage of SVR patient predicted by SCCAIC classes and prediction of SVR by RVR and EVR was performed the McNemar test to compare paired percentage. A P-value <0.05 was considered statistically significant. All analyses were performed with software SAS 9.2 for PC (SAS Institute, Cary, NC, USA).
Results
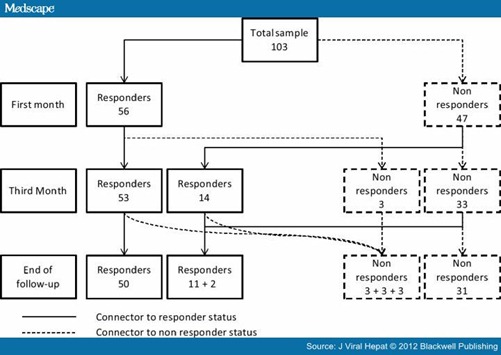
There were 63 responders (Table 1); mean age was 53.5 year (±11.9 year) for responders and 58.3 year (±10.7 year) for nonresponders; this difference resulted statistically significant (t-test = 2.1; P = 0.038) (Fig. 1). There were no statistically significant differences in the percentage of responders between men and women: 62.3% (38/61) vs 59.5% (25/42) (χ2 = 0.08; P = 0.7767).
Figure 1. Patients course from the study start to the end of follow-up.
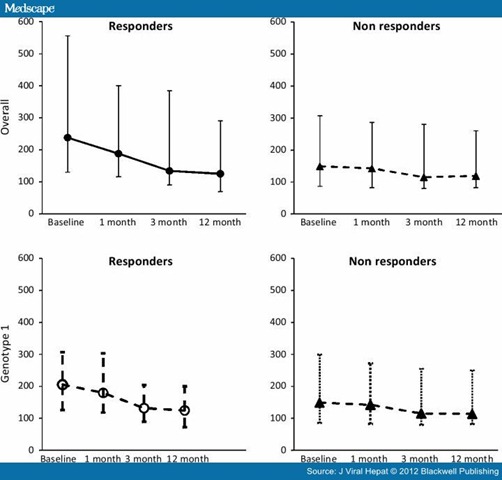
Genotype 1 was observed in 41.3% (26/63) of responders, vs 90% (36/40) of non responders: the percentage of genotype 1 was significantly different between responders and nonresponders (χ2 = 24.24; P < 0.0001). The median value of SCCA-IC by genotype and response is shown in Table 2 and profile by time of genotype 1 patients is shown in Fig. 2. Responders with all genotypes had higher levels of SCCA-IC than nonresponders. Furthermore, in responders, the level of SCCA-IC showed a tendency to decrease, whereas in nonresponders the SCCA-IC level appeared to remain the same at each time point. The median level of SCCA-IC at baseline resulted lower respect to overall median value, but profile for responders patients resulted similar (like a parallel line) until the last determination, where values for genotype 1 and overall sample resulted the same. Nonresponders patients with genotype 1 had identical value respect to overall sample, because the most part of nonresponders patients was genotype 1.
Figure 2. Median and interquartile range of SCCA-IC concentrations at each follow-up time point. In responders, the median level at each time point was less than the median at the previous time, while in nonresponders the median level was approximatively the same at each time point.
The initial value of SCCA-IC (Fig. 2) in responders was 238 AU (130–556), this decreased to 188 AU (116–400) after 1 month, to 134 AU (90–385) after 3 months and reached the value 125 AU (70–290) at the end of follow-up. In nonresponders, the initial value was 149 AU (86.5–306.5) and remained substantially unchanged after 1 month, 142.5 AU (82.5–286.5), showing a slight decrease after 3 months 115 AU (80–280) and remaining at this level until the end of follow-up: 119 AU (82–260). The difference in concentration between responders and nonresponders at the first month resulted statistically significant (P = 0.0031), while at the other time points it was not significant (P > 0.05). The decrease between consecutive time points resulted statistically significant (P < 0.001) in the responders group: the baseline-first month difference was 43 AU (15–101); first – third month, 38 AU (10–68); third month – end of the study, 14 AU (3–45). In the nonresponders group, the decrease at consecutive time points resulted significant between baseline-first month (5 AU; 0–14.5; P < 0.001), but not at subsequent time points, when the reduction was 0.5 AU (0–12.5) and 0 AU (9 to −8). Median reduction resulted significantly different between responders and nonresponders (P < 0.0001 at each comparison) at every time point. These results allowed us to conclude that the profile of reduction was different between patients that would achieve a positive SVR as compared to nonresponders.
To evaluate the effect of SCCA-IC reduction in SVR, a logistic regression model was built with the presence of SVR as dependent variable, while the independent variables were the class of SCCA-IC reduction after 1, 3 months and at the end of follow-up, genotype 1 vs others, sex (M vs F) and age (<58 vs≥58). The model resulted statistically significant (Table 3; P < 0.0001). Sex was not shown to be a statistically significant variable, while age younger than 58 increased the probability of achieving SVR (OR = 9.66; CI95% 2.21–42.15; P = 0.0025). An OR = 0.094 (CI95% 0.21–0.42; P = 0.002) was shown for genotype 1, suggesting that with this genotype patients are more likely to be nonresponders. Predictive factors of SVR were the reduction after first month, after the third month and at follow-up. No effect of interaction between time points and genotype resulted statistically significant, and this parameter was therefore removed from the model. Looking at the odds ratio, it seems that a reduction of more than 12% between the baseline value and the first time point increases by 4.82-fold the probability of SVR (95% CI 1.39–16.67; P = 0.131). A reduction between the first month and third month of more than 12% increases the probability of SVR 15-fold (3.44–66.74; P = 0.0003), and a further reduction of more than 7% until the end of the study period increases the probability of SVR 9-fold (95% CI 2.22–42.15; P = 0.043).
Taking into account only genotype 1 patients reduction in SCCA-IC between first month and baseline resulted with a borderline significativity (OR = 3.65, CI95% 0.97–13.76, P = 0.055), while reduction between third month and first month remain statistically significant (OR = 9.09, CI95% 2.085–39.69, P = 0.003).
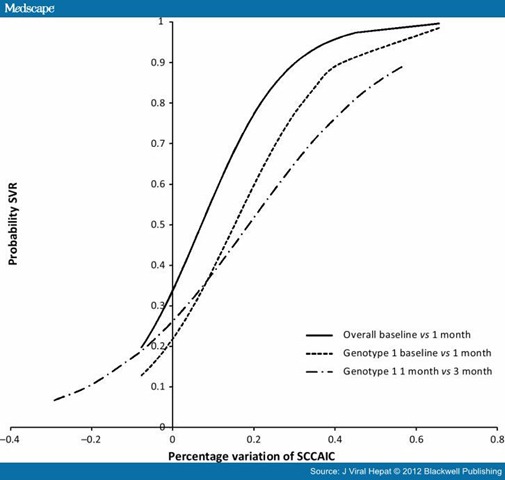
Figure 3 shows the probability of response for each value of percentage reduction in SCCA-IC between baseline and the first month for the whole sample and for genotype 1 subgroup: the probability of SVR obtained with the univariate logistic model (independent variable SVR Y/N and dependent percentage reduction in SCCA-IC as continuous variable) is increased by more than 50% for small levels of reduction starting from 7% to 8% as compared to the baseline value. For genotype 1, subgroup instead the probability of being a responder patient at the end of follow-up resulted more than 50% for higher value of reduction (at least 15%). Positive predictive value of SVR of SCCA-IC reduction was 69.8% (44 with reduction/63 SVR patients), while RVR 79.4% (50 RVR/63 SVR) and EVR 96.8% (61 EVR/63 SVR). There was a statistically significant difference between predictive value of class of SCCA-IC and EVR (McNemar test 17.0, P < 0.0001), but not respect to RVR (McNemar test 1.8, P = 0.179). This results are confirmed in the subgroup of genotype 1 patients: predictive value of class of SCCA-IC reduction was 65.4% (17 with reduction/26 SVR), while for RVR was 69.3% (18/26), that resulted not significantly different (P = 0.76) and for EVR 96.1% (25/26) significantly different respect to class of SCCA-IC (P = 0.0047) (Table 4).
Figure 3. Probability of being a responder as a function of the SCCA-IC reduction. The x-axis shows the percentage variation obtained as SCCA-IC value at baseline less the value at the first month divided by the baseline value.
Discussion
In this study, we have demonstrated that a decreased serum concentration of SCCA-IC after 4 weeks of antiviral therapy is an independent prognostic factor of therapeutic response. We base this conclusion on the following data: (i) the reduction in SCCA-IC was significantly different between patients achieving SVR or not; (ii) the profile of SCCA-IC is different between responders and nonresponders even with different genotypes; (iii) If the reduction is evaluated as percentage, the effect of the decrease between baseline and the first month value becomes even more noticeable.
The model evaluating the effect of predictors such as genotype and SCCA-IC reduction at each time point was shown to be well able to predict the response. The reduction at the first time point seems to be a good predictor with low variability, and the probability allows us to conclude that if a reduction is observed at the first follow-up visit the patient will achieve a positive clinical response.
The genotype shows a strong influence on obtaining a SVR, but does not affect the conclusions about SCCA-IC, that can therefore be considered a useful marker for monitoring patients when measured at each visit: before starting therapy and after the first and second follow-up visit. Thus, if SCCA-IC serum levels reduce during therapy, the patient will achieve SVR. Should be noticed that genotype 1 subgroup has less probability to be a responder, but even in this group if a reduction in SCCA-IC level is observed a SVR could be obtained.
In a previous work by Giannini et al.,[6] the authors reported that a higher SCCA-IC, but not SCCA, serum concentrations reduction in patients with liver cirrhosis was observed in responders to antiviral therapy. Probably because of the small sample size, they could not make an appropriate test of the significance of the reduction with respect to the future response. Moreover, in that work the genotype was not evaluated together with SCCA-IC in a multivariate model. In this article, instead, the authors focused on the definition of a parameter to predict SVR, so both genotype and repeated reductions of the marker have been evaluated in a multivariate model. This confirmed the importance of the reduction of the marker, more than the value of the marker itself, as an independent variable, specifically with respect to the genotype. Our data demonstrate that patients with SVR have a decreasing profile according to genotype. This suggests that SVR could be predicted by genotype, as already known, but that important information on a positive response can be gained if there is a reduction more than 12% at the first month as compared to the baseline value.
Therefore, an antigen immuno-complex such as SCCA-IC can predict the clinical outcome. It remains to be seen what molecular mechanism is related to the decrease in SCCA-IC, to explain its significance as a prognostic factor.
In other studies, SCCA-IC was reported as a new biomarker for early detection of HCC. However, while the sensitivity performance was very encouraging, the specificity was disappointing. In particular, patients without HCC but with underlying chronic liver disease had unexplainably high serum levels of SCCA-IC.[8,11] We hypothesize that SCCA-IC could be related to the liver fibrosis/inflammation status.[12] In fact, we detected increased levels of SCCA-IC in patients with systemic sclerosis (SS). In particular. among these patients, we found that those with fibrotic involvement of the lung displayed the highest serum levels of SCCA-IC, as compared with the other subsets of SS patients with a different clinical outcome.[13] Therefore, it is likely that SCCA-IC serum levels reflect the fibrosis-immunity-related status, which could explain why this parameter is a prognostic factor independently of the genotype or the RVR.
In conclusion, we report a new prognostic factor that can predict the therapeutic response in patients with chronic hepatitis C undergoing antiviral therapy. SCCA-IC can be easily measured using a commercially available ELISA kit. Finally, we suggest that being an independent prognostic factor, SCCA-IC may be useful to refine patients selection, discriminating those patients that will benefit from antiviral therapy and thus optimizing the cost-benefit ratio.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49: 1335– 1374.
- McHutchison JG, Lawitz EJ, Shiffman ML et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361: 580–593.
- Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006; 55: 1350–1359.
- Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol 2011; 55: 69–75.
- EASL. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011; 55: 245–264.
- Giannini EG, Basso M, Bazzica M et al. Successful antiviral therapy determines a significant decrease in squamous cell carcinoma antigen-associated (SCCA) variants' serum levels in anti-HCV positive cirrhotic patients. J Viral Hepat 2010; 17: 563–568.
- Beneduce L, Castaldi F, Marino M et al. Squamous cell carcinoma antigen-immunoglobulin M complexes as novel biomarkers for hepatocellular carcinoma. Cancer 2005; 103: 2558–2565.
- Pontisso P, Quarta S, Caberlotto C et al. Progressive increase of SCCA-IgM immune complexes in cirrhotic patients is associated with development of hepatocellular carcinoma. Int J Cancer 2006; 119: 735–740.
- Giannelli G, Antonaci S. New frontiers in biomarkers for hepatocellular carcinoma. Dig Liver Dis 2006; 38: 854–859.
- Giannelli G, Fransvea E, Trerotoli P et al. Clinical validation of combined serological biomarkers for improved hepatocellular carcinoma diagnosis in 961 patients. Clin Chim Acta 2007; 383: 147–152.
- Beale G, Chattopadhyay D, Gray J et al. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer 2008; 8: 200.
- Trerotoli P, Fransvea E, Angelotti U et al. Tissue expression of Squamous Cellular Carcinoma Antigen (SCCA) is inversely correlated to tumor size in HCC. Mol Cancer 2009; 8: 29.
- Giannelli G, Iannone F, Fransvea E, Chiala A, Lapadula G, Antonaci S. Squamous cellular carcinoma immunocomplexed is increased in scleroderma patients with lung fibrosis. Clin Exp Rheumatol 2007; 25: 794–795.
No comments:
Post a Comment