C. Koh, T. Heller, V. Haynes-Williams, K. Hara, X. Zhao, J. J. Feld, D. E. Kleiner, Y. Rotman, M. G. Ghany, T. J. Liang, J. H. Hoofnagle
Aliment Pharmacol Ther. 2013;37(9):887-894.
Abstract and Introduction
Abstract
Background Although the short-term benefits of a sustained virological response (SVR) to interferon-based therapies of chronic hepatitis C (CHC) are well known, the long-term consequences of SVR are less clear.
Aim To assess changes in markers of disease activity and fibrosis in patients followed up to 23 years post-SVR.
Methods The first 103 SVR patients (from 1984 to 2003) at the National Institutes of Health Clinical Center were evaluated. Serum markers before treatment and at the last visit were compared. Evaluations after 2007 included transient elastography (TE).
Results Of 103 patients, three subsequently relapsed 0.7, 6.3 and 6.5 years post therapy. The remaining 100 patients (56 men, mean age 56 years) maintained SVR at final follow-up. No patients developed hepatic decompensation, but one with pre-treatment cirrhosis died 12 years post SVR of hepatocellular carcinoma. In comparison to pre-treatment values, markers improved at follow-up, including mean ALT (152–27 U/L), AST (87–24 U/L), alkaline phosphatase (78–69 U/L), IgG (1463–1113 mg/dL), platelet count (209 000–239 000/μL) and AST to platelet count ratio index (APRI: 1.31–0.33). TE was performed in 69 patients and was normal (<7.0 kPA) in 60%, moderately elevated (7.1–13.8) in 31% and cirrhotic range (>13.8) in 9%. TE and platelet counts at follow-up correlated with fibrosis on pre-treatment liver biopsy (P < 0.001).
Conclusions In 97% of patients with CHC, SVR is durable without evidence of disease progression, although some degree of hepatic fibrosis may persist and patients with pre-treatment cirrhosis are at continuing low risk for hepatocellular carcinoma.
Introduction
Chronic hepatitis C virus (HCV) infection is estimated to affect 180 million persons worldwide and at least 3.2 million Americans.[1, 2] This disease is the most common cause of chronic hepatitis and cirrhosis, end-stage liver disease and hepatocellular carcinoma in the United States and the developed world.[3, 4] It is also the single most common reason for liver transplantation.[4] Therapy for HCV has evolved during the last 20 years from use of interferon alone to the combination of interferon and ribavirin followed by the combination of peginterferon with ribavirin.[5–7] Recently direct-acting antiviral agents have been developed for HCV that have increased the response rate substantially.[8–13] The endpoint for assessing efficacy of antiviral therapy has been the loss of HCV RNA from serum which, if sustained for at least 6 months after stopping treatment, is referred to as a sustained virological response (SVR).[14] The durability of a 6-month SVR is above 95%, but the long-term clinical benefits of this outcome have not been well defined.[15, 16]
Although achievement of an SVR has been associated with clinical, laboratory and histological improvements in chronic HCV, this endpoint is a surrogate for the ultimate aim of therapy, which is prevention of progression to cirrhosis, end-stage liver disease, hepatocellular carcinoma and death from liver disease.[17–26] These 'hard' endpoints, however, generally take years or decades to evolve and following patients randomised to treatment or observation with these dire outcomes has not been practical and has been considered ethically untenable. Thus, while SVR is associated with improvements in serum aminotransferase levels and liver histology, its role in preventing progression of disease and disability or death from chronic liver disease is uncertain and currently controversial.
At the Clinical Center of the National Institutes of Health (NIH), we have conducted a series of prospective controlled and uncontrolled studies of therapy of chronic HCV beginning in 1984.[26–31] All patients who achieved an SVR as a part of these studies have been followed on a long-term basis to assess the natural history and outcome of this virological response. As a consequence, we have a cohort of SVR patients that have been followed up for up to 23 years. We report the follow-up of the initial 103 patients who achieved an SVR between the years 1986 and 2003.
Materials and Methods
Patients
All patients who achieved a 6-month post-treatment SVR in clinical research protocols conducted by the Liver Diseases Branch, NIDDK between 1984 and 2003 were included in this analysis. Of the 262 patients enrolled, 103 achieved an SVR. Thereafter, many patients were followed on a regular basis. Starting in 2007, patients who had not returned in the previous 2 years were asked to return for a medical evaluation, blood tests, abdominal ultrasound and ultrasound transient elastography. The initial protocols included studies of interferon alfa-2b alone for 6 or 12 months,[26–29] escalating doses of interferon alfa-2b for 12 months, and the combination of standard interferon alfa-2b or peginterferon alfa-2a with ribavirin for 6–12 months.[29–31] All patients gave written informed consent for the initial trials as well as for long-term follow-up and transient elastography in a protocol, which was approved by the NIDDK Institutional Review Board and registered in ClinicalTrials.gov (#NCT00001971). All authors had access to the study data and had reviewed and approved the final manuscript.
Laboratory Tests
At the time of last follow-up, patients were tested for liver biomarkers including alanine and aspartate aminotransferase (ALT, AST), alkaline phosphatase, gamma glutamyl-transpeptidase (GGT), immunoglobulin levels (IgG, IgA, IgM), rheumatoid factor, alpha-fetoprotein and platelet counts. Results of these markers before therapy were available from most patients. Serum HCV RNA was tested by quantitative and qualitative reverse transcriptase polymerase chain reaction (Amplicor; Roche Molecular Systems, Pleasanton, CA, USA) with a lower limit of sensitivity of 100 viral copies/mL (50 IU/mL). HCV genotyping was done using reverse hybridisation (INNO-LiPA; Innogenetics, Ghent, Belgium). HCV RNA testing before, during and immediately after therapy was performed using assays that were available at the time and were not always of similar specificity and sensitivity. Virological testing from the initial studies (done between 1984 and 1992) was done on serum samples, stored at −80 °C.[29] In patients who relapsed, stored serum samples (−80 °C) were retrieved and retested for HCV RNA at the time of SVR using the Amplicor system to confirm the absence of HCV RNA.
All except three patients underwent a pre-treatment liver biopsy with interpretation by one hepatopathologist (DEK) using the Ishak modification of the HAI scoring system for activity and fibrosis.[32]
Transient Elastography
Patients evaluated after 2007 underwent ultrasound transient elastography (TE) performed via FibroScan (Echosens, Paris) after informed consent.[33] Elastography was done by individuals specifically trained in the technique. At least 10 determinations were made and median results were expressed in kilopascals (kPA). Values range from 2.5 to 75 kPA with values of <7.0 being normal and suggesting no or mild hepatic fibrosis, values of 7.1–13.7 suggesting moderate to advanced fibrosis and values of >13.8 suggesting cirrhosis.
Statistical Analysis
Continuous variables were reported as mean (±standard deviation), and categorical variables as percentages. Mean and proportion of abnormal values, differences of baseline ALT, AST, GGT, alkaline phosphatase, total and direct bilirubin, IgG, albumin, alpha-fetoprotein, rheumatoid factor and platelets and the most recent follow-up values were compared via paired student's t-test for continuous variables or McNemar's test for categorical variables. As a sensitivity analysis, regression analysis was used to explore the effect in time between baseline to follow-up and mean differences between baseline and follow-up values. Cumulative incidence of relapse was plotted using the Kaplan–Meier method. In comparing pre-treatment liver biopsy scores with follow-up TE, the McNemar's exact test for association was used. Significance was accepted at a P-value of less than 0.05. Data analysis was performed using sas, Version 9.1.3 (SAS Institute Inc., Cary, NC, USA) software.
Results
Among 262 patients treated in five clinical research protocols between 1984 and 2003, 103 had an SVR and comprised the analysis cohort for this study. These patients were followed up for a median time of 7.5 years (6 months to 23 years) after SVR and 89 were seen and evaluated after 2007. The duration of follow-up after SVR was less than 5 years in 27 patients, 5–10 years in 58 patients, and greater than 10 years in 12 patients. Of this cohort, three patients were found to be HCV RNA positive at the time of follow-up and were considered late relapsers. The estimated time to relapse was 0.67, 6.3 and 6.5 years after stopping therapy. Thus, the relapse free rate was 96% at an average of 7.6 years after therapy (Figure 1). The three HCV RNA positive patients appeared to have suffered a relapse rather than re-infection, as the recurrent virus was almost identical to the pre-treatment sequence strains (Supplemental Table S1, published online).[34] None had ongoing risk factors for hepatitis C or a clear predisposition for relapse (i.e. corticosteroids, chemotherapy or immunosuppression).
Figure 1.
Kaplan–Meier analysis of proportion of patients without virological relapse. Three of 103 patients became HCV RNA positive after having achieved an SVR after interferon-based therapy. At 7.2 years, the relapse free rate was 96%.The remaining 100 patients included 56 men and 44 women, 88 whites, 4 African Americans and 8 Asians, with an average age of 55.6 years (). Twenty-one received standard interferon alone, 55 interferon and ribavirin and 24 peginterferon and ribavirin.
Table 1. Clinical features of 100 patients with sustained virological response
| Feature | Number |
|---|---|
| Number of men/women (%) | 56(56%)/44(44%) |
| Median duration of follow-up in years (range) | 7.5 (0.6–23.0) |
| Mean age at last visit (range) in years | 55.6 (16.7–84.2) |
| Number of deaths/liver-related deaths | 6/1 |
| Race | |
| Number of Caucasians (%) | 88 (88%) |
| Number of African Americans (%) | 4 (4%) |
| Number of Asians (%) | 8 (8%) |
| Number of patients with HCV genotype (%) | |
| 1 | 45 (45%) |
| 2 | 35 (35%) |
| 3 | 18 (18%) |
| Others | 2 (2%) |
| Number of patients with pre-treatment Ishak fibrosis score (%) | |
| 0–2 | 62 (64%) |
| 3–4 | 25 (26%) |
| 5–6 | 10 (10%) |
| Number of patients undergoing treatment (1985–2003) (%) | |
| Interferon alfa-2b | 21 (21%) |
| Interferon alfa-2b & Ribavirin | 55 (55%) |
| Peginterferon alfa-2a & Ribavirin | 24 (24%) |
In follow-up, six of the 100 patients died but only one died of a HCV-related cause, hepatocellular carcinoma resulting in death 12.5 years after SVR. This patient had biopsy proven cirrhosis before the course of therapy with interferon and ribavirin that led to an SVR. The causes of death in the remaining five patients included Huntington's chorea, cerebrovascular disease, septicaemia from chronic osteomyelitis, pneumonia and cardiac arrest all of which occurred more than a year after therapy and none of which were considered related to hepatitis C or its treatment. No patient developed decompensated liver disease, jaundice, ascites, variceal haemorrhage, hepatic encephalopathy or required liver transplantation.
Follow-up testing was available from all patients 12–264 months after completion of therapy. Serum ALT levels were normal in 90% and the average value was 27 U/L, which was significantly less than before therapy (152 U/L) ( and ). Of the remaining 10% of patients with abnormal follow-up ALT levels, values were minimally or modestly elevated and were less than twice the upper limit of the normal range in nine. All 10 patients with ALT elevations had gained weight; four were overweight and five were obese. Abdominal ultrasound demonstrated steatosis in all six who underwent imaging during follow-up (Supplemental Table S2, published online).
Table 2. Mean results of routine laboratory values taken before therapy and at the time of last follow-up evaluation
| Laboratory test | Initial value (range) | Final value (range) | P-value |
|---|---|---|---|
| ALT (U/L) | 152 (16–459) | 27 (10–72) | <0.0001 |
| AST (U/L) | 87 (18–296) | 24 (14–44) | <0.0001 |
| GGT (U/L) | 47 (11–176) | 28 (5–91) | <0.0001 |
| ALP (U/L) | 78 (37–237) | 69 (28–238) | <0.0001 |
| Total bilirubin (mg/dL) | 0.7 (0.3–2.3) | 0.8 (0.1–2.7) | 0.055 |
| Albumin (g/dL) | 4.2 (3.0–5.0) | 4.1 (3.1–5.2) | 0.016 |
| Alpha-fetoprotein (ng/mL) | 4.6 (1.2–26.6) | 2.9 (1.2–9.0) | <0.0001 |
| IgG (mg/dL) | 1463 (518–2580) | 1113 (605–2090) | <0.0001 |
| Platelets (×1000/μL) | 209 (73–384) | 239 (56–457) | <0.0001 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase.
Analysis by paired student's t-test.
Table 3. Proportion of patients with abnormal laboratory results before therapy and at the time of last follow-up evaluation
| Laboratory test | Percentage abnormal | P-value | |
|---|---|---|---|
| Initial | Final | ||
| ALT | 83 | 10 | <0.0001 |
| AST | 79 | 5 | <0.0001 |
| Direct bilirubin | 47 | 26 | 0.048 |
| Rheumatoid factor | 38 | 19 | 0.0043 |
| Platelet count | 24 | 10 | 0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Analysis by McNemar's test.
At the time of final follow-up there were also significant improvements in mean AST (87–24 U/L), alkaline phosphatase (78–69 U/L), IgG (1463–1113 mg/dL), alpha-fetoprotein (4.6–2.9 ng/mL), GGT (47–28 U/L), platelet count (209 000–239 000/μL) and rheumatoid factor (38–19% positive). Based on sensitivity analysis using regression models, patients with longer duration of follow-up had greater mean changes of ALT, AST, total and direct bilirubin, IgG and albumin values between baseline and follow-up (data not shown). Serum total bilirubin and albumin levels did not change appreciably, but were largely normal even before therapy. Direct bilirubin improved slightly although not significantly overall. Before therapy, 47% of patients had a direct bilirubin level of greater than 0.2 mg/dL (range: 0.0–0.4 mg/dL); at the time of last follow-up testing, only 26% had abnormal values (range: 0.1–0.4 mg/dL) (). On follow-up evaluation, no patient had an abnormal prothrombin time. Because the methodology and normal range for the prothrombin time varied over the period of observation, a comparison of initial and final values was not possible.
Table 3. Proportion of patients with abnormal laboratory results before therapy and at the time of last follow-up evaluation
| Laboratory test | Percentage abnormal | P-value | |
|---|---|---|---|
| Initial | Final | ||
| ALT | 83 | 10 | <0.0001 |
| AST | 79 | 5 | <0.0001 |
| Direct bilirubin | 47 | 26 | 0.048 |
| Rheumatoid factor | 38 | 19 | 0.0043 |
| Platelet count | 24 | 10 | 0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Analysis by McNemar's test.
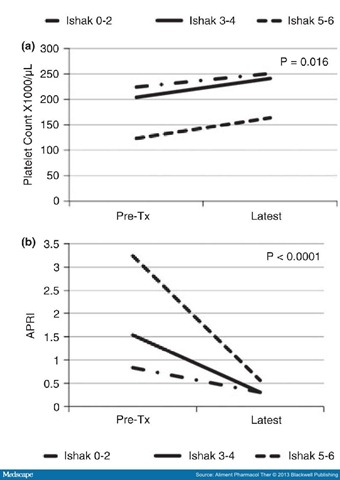
The calculated APRI was elevated (>0.8) in 53% of patients before treatment and averaged 1.31. In follow-up, the APRI was normal in all patients and averaged 0.33, which was significantly less than pre-treatment values (P < 0.001) (Figure 2).
Figure 2.
Mean platelet counts (a) and AST-to-platelet ratio index values (APRI: b) before therapy (Pre-Tx) and at the time of last follow-up evaluation in 100 patients with a sustained virological response stratified by degree of hepatic fibrosis on pre-treatment liver biopsy. Patients were categorised into three groups based upon pre-treatment Ishak fibrosis scores of 0–2 (no fibrosis to portal fibrosis only), 3–4 (bridging hepatic fibrosis) and 5–6 (early and complete cirrhosis).Transient elastography was attempted in 75 patients at follow-up, but was successful in only 69 (). In this group, 59% had stiffness scores of ≤7.0 kPA (considered normal), 32% had values between 7.1 and 13.8 (suggestive of moderate to advanced fibrosis), and 9% had values above 13.8 kPA (suggestive of cirrhosis).
Table 4. Association of initial Ishak fibrosis score from initial, pre-treatment liver biopsy and transient elastography stiffness score at the time of final follow-up evaluation
| Baseline Ishak score | Number of patients | Fibroscan on follow-up | P-value | ||
|---|---|---|---|---|---|
| ≤7.0 | 7.1–13.8 | >13.8 | |||
| 0–2 | 45 | 28 (62%) | 16 (36%) | 1 (2%) | 0.0006 |
| 3–4 | 17 | 12 (71%) | 4 (23%) | 1 (6%) |  |
| 5–6 | 7 | 1 (14%) | 2 (29%) | 4 (57%) |  |
| Total | 69 | 41 (59%) | 22 (32%) | 6 (9%) |  |
Analysis by McNemar's test.
Liver biopsy had been performed on 97 patients before therapy and demonstrated cirrhosis (Ishak fibrosis 5–6) in 10, bridging fibrosis (Ishak 3–4) in 25 and no fibrosis or portal fibrosis only (Ishak 0–2) in 62. Analysis of TE stiffness values by initial hepatic fibrosis scores demonstrated that most patients (6 of 7) with pre-treatment cirrhosis had high TE values at follow-up 3.1–23 years after an SVR. In contrast, abnormal TE values were found in only 38% of those with minimal fibrosis and 29% with bridging fibrosis before treatment.
Platelet counts were low (<160 000/μL) in 24% of patients before therapy including 80% of those with cirrhosis. In follow-up, the average platelet count in the 100 patients increased (from 209 000 to 239 000/μL) and was in the normal range in 90% ( and ). Platelet counts were generally normal in patients with no or mild fibrosis before therapy but even in these individuals, the average platelet count increased after SVR. Improvement in platelet count was most marked in patients with cirrhosis (123 000–164 000/μL) and also increased similarly in those with moderate to advanced fibrosis (204 000–241 000/μL) (Figure 2a). Importantly, the platelet count at the time of TE evaluation correlated with liver stiffness scores. Thus, the average platelet count in patients with normal TE scores was 219 902/μL, compared with 212 455/μL in those with TE scores between 7.1–13.8 and 147 333/μL in those with TE scores in the cirrhotic range of >13.8 kPA (P = 0.0248). In contrast, the APRI values correlated with the degree of hepatic fibrosis as assessed by liver biopsy before treatment, but were within the normal range in all patients at the time of follow-up evaluation regardless of initial Ishak scores or follow-up TE values (Figure 2b).
Table 2. Mean results of routine laboratory values taken before therapy and at the time of last follow-up evaluation
| Laboratory test | Initial value (range) | Final value (range) | P-value |
|---|---|---|---|
| ALT (U/L) | 152 (16–459) | 27 (10–72) | <0.0001 |
| AST (U/L) | 87 (18–296) | 24 (14–44) | <0.0001 |
| GGT (U/L) | 47 (11–176) | 28 (5–91) | <0.0001 |
| ALP (U/L) | 78 (37–237) | 69 (28–238) | <0.0001 |
| Total bilirubin (mg/dL) | 0.7 (0.3–2.3) | 0.8 (0.1–2.7) | 0.055 |
| Albumin (g/dL) | 4.2 (3.0–5.0) | 4.1 (3.1–5.2) | 0.016 |
| Alpha-fetoprotein (ng/mL) | 4.6 (1.2–26.6) | 2.9 (1.2–9.0) | <0.0001 |
| IgG (mg/dL) | 1463 (518–2580) | 1113 (605–2090) | <0.0001 |
| Platelets (×1000/μL) | 209 (73–384) | 239 (56–457) | <0.0001 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase.
Analysis by paired student's t-test.
Table 3. Proportion of patients with abnormal laboratory results before therapy and at the time of last follow-up evaluation
| Laboratory test | Percentage abnormal | P-value | |
|---|---|---|---|
| Initial | Final | ||
| ALT | 83 | 10 | <0.0001 |
| AST | 79 | 5 | <0.0001 |
| Direct bilirubin | 47 | 26 | 0.048 |
| Rheumatoid factor | 38 | 19 | 0.0043 |
| Platelet count | 24 | 10 | 0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Analysis by McNemar's test.
Discussion
The initial 5 of 10 patients treated with antiviral therapy in 1986 at the NIH for chronic HCV achieved an SVR and had both biochemical and histological evidence of improvement in the year following treatment.[26] These five patients have now been followed up for more than 20 years and remain HCV RNA-negative and have normal or near-normal serum enzyme levels.[28] Liver biopsies on these patients 10 years after initial therapy showed resolution of the disease activity and regression of fibrosis in some.[35] After this initial study, patients at the NIH were enrolled in various therapeutic trials for chronic hepatitis C. As of 2003, a total of 103 patients had achieved an SVR, all in response to interferon-based therapy. The duration of subsequent follow-up in these 103 patients varied from a few months to as long as 23 years. In this cohort, three patients relapsed, and the remaining 100 patients had markedly improved liver tests at the time of follow-up evaluation and none had clinical evidence of advanced cirrhosis, hepatic decompensation or end-stage liver disease. These findings indicate that an SVR from interferon-based therapies for chronic HCV is usually durable and associated with improvement in biomarkers of disease, a favourable long-term prognosis and lack of evidence of progression of liver disease.
Similar findings after SVR in chronic HCV have been published in other cohorts.[15–20, 36–39] However, the current analysis extends this experience to more than 20 years after therapy. Importantly, while patients who achieved an SVR did not develop progressive liver disease, at least one case of HCC still occurred. In this cohort, one patient who had cirrhosis before treatment developed HCC despite having had an SVR 12 years previously. The occurrence of HCC after SVR has been reported in several cohorts, although the rate of liver cancer appears to be far less than occurs among untreated patients with advanced fibrosis or cirrhosis due to chronic hepatitis C.[19, 20, 36, 37] Such findings suggest that patients with an SVR should continue to have regular surveillance for HCC if they had histological evidence of cirrhosis before treatment.
A shortcoming of this study is the lack of a control group of patients with chronic hepatitis C who were not treated or a comparison group of patients who were treated but did not achieve an SVR. However, it was not feasible or considered ethical to randomise patients to therapy vs. no therapy and follow them for an indefinite period. In early controlled trials of interferon for hepatitis C, some patients were not treated for the initial 1–2 years after randomisation.[27] However, the controls from those studies were subsequently offered therapy on an open-label basis and some achieved an SVR and are a part of this analysis. Since 1992 and the approval of interferon as therapy of hepatitis C, all large 'controlled' trials of treatment have compared one interferon-based regimen to another and patients were not given placebo or randomised to no therapy.
Another approach to assessment of the possible benefit of an SVR is to compare patients who achieve an SVR to those who relapse or do not respond. However, multiple studies have shown that patients who have an SVR have a durable loss of HCV RNA and are less likely to have advanced fibrosis or cirrhosis.[5–7, 27] For these reasons, such comparisons require careful balancing of risk factors. Among the 262 patients treated in at this centre, the majority of the non responders were retreated at one time or another, with differing regimens and often at different institutions.
Use of transient elastography, a non-invasive marker for hepatic fibrosis, suggested that 41% of the cohort had residual evidence of fibrosis at the time of last follow-up 3–23 years after SVR. The elevated elastography scores were not associated with residual abnormalities of serum aminotransferase levels but were more likely to be abnormal in patients who had advanced fibrosis or cirrhosis before treatment. This suggests that some degree of fibrosis persists despite the resolution of disease activity as assessed by serum enzymes. Slight decreases in platelet counts at the time of final follow-up also correlated with the initial liver histology. Elastography scores were not available from before treatment, but improvements in other markers of advanced disease (platelet count, direct bilirubin, immunoglobulin levels) suggest that SVR may be associated with subsequent improvement in portal hypertension and perhaps partial regression of fibrosis. These findings suggest that the greatest benefit from successful eradication of HCV may be in non-cirrhotic patients, but that even patients with advanced disease, gain a benefit from treatment.
In summary, with continued follow-up of patients with chronic hepatitis C for up to 23 years after achieving sustained clearance of HCV RNA, progressive liver disease was not seen. Among patients who had advanced fibrosis and cirrhosis before being treated, evidence from platelet counts and transient elastography suggested the persistence of some degree of hepatic fibrosis and a low but continued risk for HCC.
References
-
Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology (Baltimore, MD) 2009; 49: 1335–74.
-
Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144: 705–14.
-
Williams R. Global challenges in liver disease. Hepatology (Baltimore, MD) 2006; 44: 521–6.
-
Kim WR. The burden of hepatitis C in the United States. Hepatology(Baltimore, MD) 2002; 36: S30–4.
-
Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958–65.
-
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975–82.
-
Hadziyannis SJ, Sette H Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140: 346–55.
-
Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet 2010; 376: 705–16.
-
Poordad F, McCone J Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl JMed 2011; 364: 1195–206.
-
Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. NEngl J Med 2011; 364: 1207–17.
-
Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009; 360: 1839–50.
-
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364: 2405–16.
-
Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364: 2417–28.
-
Lindsay KL. Introduction to therapy of hepatitis C. Hepatology (Baltimore, MD) 2002; 36: S114–20.
-
Swain MG, Lai MY, Shiffman ML, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 2010; 139: 1593–601.
-
Formann E, Steindl-Munda P, Hofer H, et al. Long-term follow-up of chronic hepatitis C patients with sustained virological response to various forms of interferon-based anti-viral therapy. Aliment Pharmacol Ther 2006; 23: 507–11.
-
Bernstein D, Kleinman L, Barker CM, Revicki DA, Green J. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology (Baltimore, MD) 2002; 35: 704–8.
-
Camma C, Di BD, Schepis F, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology (Baltimore, MD) 2004; 39: 333–42.
-
Coverdale SA, Khan MH, Byth K, et al. Effects of interferon treatment response on liver complications of chronic hepatitis C: 9-year follow-up study. AmJ Gastroenterol 2004; 99: 636–44.
-
George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology(Baltimore, MD) 2009; 49: 729–38.
-
Hassanein T, Cooksley G, Sulkowski M, et al. The impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol 2004; 40: 675–81.
-
Hung CH, Lee CM, Lu SN, et al. Longterm effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat 2006; 13: 409–14.
-
Marcellin P, Boyer N, Gervais A, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferonalpha therapy. Ann Intern Med 1997; 127: 875–81.
-
Veldt BJ, Saracco G, Boyer N, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut 2004; 53: 1504–8.
-
John-Baptiste AA, Tomlinson G, Hsu PC, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol 2009; 104: 2439–48.
-
Hoofnagle JH, Mullen KD, Jones DB, et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med 1986; 315: 1575–8.
-
Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebocontrolled trial. N Engl J Med 1989; 321: 1506–10.
-
Shindo M, Di Bisceglie AM, Cheung L, et al. Decrease in serum hepatitis C viral RNA during alpha-interferon therapy for chronic hepatitis C. AnnIntern Med 1991; 115: 700–4.
-
Hoofnagle JH, Ghany MG, Kleiner DE, et al. Maintenance therapy with ribavirin in patients with chronic hepatitis C who fail to respond to combination therapy with interferon alfa and ribavirin. Hepatology(Baltimore, MD) 2003; 38: 66–74.
-
Feld JJ, Lutchman GA, Heller T, et al. Ribavirin improves early responses to peginterferon through improved interferon signaling. Gastroenterology 2010; 139: e4.
-
Rotman Y, Borg BB, Soza A, et al. Low- and standard-dose peginterferon alfa-2a for chronic hepatitis C, genotype 2 or 3: efficacy, tolerability, viral kinetics and cytokine response. Aliment Pharmacol Ther 2010; 31: 1018–27.
-
Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696–9.
-
Castera L, Forns X, Alberti A. Noninvasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008; 48: 835–47.
-
Hara KKC, Sakiani S, Rivera M, Liang TJ, Hoofnagle JH, Heller T. Important determinants of 5' untranslated region (UTR) in hepatitis C virus (HCV) genotyping: clinical implications for today and the future [abstract]. In: The62nd Annual Meeting of the AmericaAssociation for the Study of Liver Diseases 2011; Nov 4–8; San Francisco, CA.
-
Lau DT, Kleiner DE, Ghany MG, Park Y, Schmid P, Hoofnagle JH. 10-Year follow-up after interferon-alpha therapy for chronic hepatitis C. Hepatology(Baltimore, MD) 1998; 28: 1121–7.
-
Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology (Baltimore, MD) 2007; 45: 579–87.
-
Giannini EG, Basso M, Savarino V, Picciotto A. Sustained virological response to pegylated interferon and ribavirin is maintained during longterm follow-up of chronic hepatitis C patients. Aliment Pharmacol Ther 2010; 31: 502–8.
-
Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol 2010; 8: 280–8.
-
Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med 2007; 147: 677–84.
Guarantor of the article
Christopher Koh.
Author contributions
Study concept & design: CK, TH, JHH; Acquisition of data: CK, TH, VH, JJF, YR, MGG, TJL, JHH; analysis/interpretation: CK, DEK, KH, TH, JHH; Drafting: CK; Critical revision: TH, VH, KH, JJF, XZ, DEK, JHH, MGG, YR, TJL; Statistical analysis: CK, XZ; Technical Support: None; Supervision: JHH, TH, TJL.
All authors approved the final version of the manuscript.
Aliment Pharmacol Ther. 2013;37(9):887-894. © 2013 Blackwell Publishing

No comments:
Post a Comment