Provided by NATAP
Research Letter | JAMA December 25, 2013
Marcus A. Bachhuber, MD1; Chinazo O. Cunningham, MD, MS1
1Division of General Internal Medicine, Albert Einstein College of Medicine, Bronx, New York
---------------------------------------------------------------
Why Aren't More Drug Treatment Centers Screening for HIV and Hep C?
healthline Written by Shawn Radcliffe | Published on December 24, 2013
Screening for potentially deadly infectious diseases has dropped at for-profit opioid treatment programs over the past decade.
In spite of government recommendations for more widespread screening, the percentage of for-profit opioid treatment programs offering on-site testing for HIV, the hepatitis C virus, and other sexually transmitted infections (STIs) has dropped over the past decade.
This decrease in screening may unnecessarily delay the diagnosis and treatment of people enrolled in these programs and increase the chances that they will pass on infectious diseases to others.
"Opioid dependence-addiction to heroin, prescription painkillers, or both-is a very well-known risk factor for HIV, hepatitis C virus, and sexually transmitted infections," says Marcus A. Bachhuber, M.D., of Albert Einstein College of Medicine, co-author of a Dec. 25 letter on STI testing at treatment centers in the journal JAMA.
Drop in Screening for Infectious Diseases
Using data from an annual survey sent to the directors of drug treatment facilities in the U.S., the researchers found stark differences among the levels of screening offered at public, nonprofit, and for-profit opioid treatment centers.
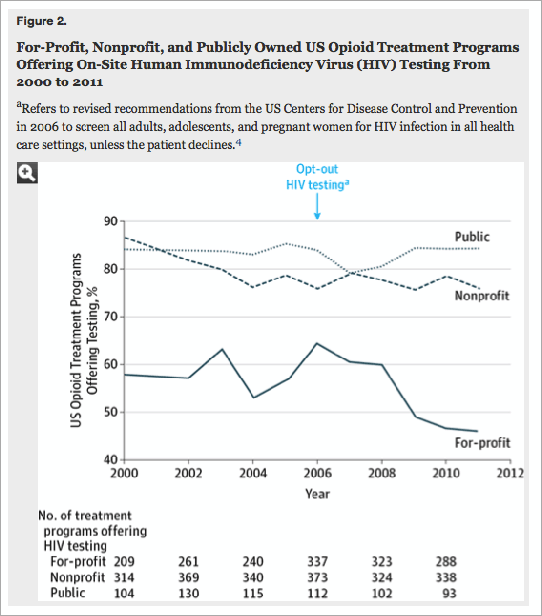
While more than 75 percent of public programs offered on-site testing for HIV, hepatitis C, and STIs during the 11 year study period, the percentage of for-profit programs screening for these infections declined during that time. From 2000 to 2011, on-site screening for HIV dropped by 20 percent in for-profit programs, while screening for hepatitis C declined by 13 percent and STIs by 23 percent.
Rise in Number of For-Profit Programs
Opioid treatment programs "were among the first venues to offer HIV testing," the study authors write, "and are more likely to offer HIV, STI, and HCV [hepatitis C virus] testing than other drug treatment programs."
These strengths, however, are offset by the failure of many for-profit programs to offer on-site screening for potentially deadly infectious diseases, coupled with a rise in the number of these programs nationwide.
Of the more than 1,000 opioid treatment programs in the U.S.-which provide treatment to more than 300,000 people each year-54 percent were for-profit in 2011, up from 43 percent in 2000.
Opt-Out Screening Has Little Effect
In 2006, the Centers for Disease Control and Prevention revised its stance on HIV screening to include opt-out testing for patients in all healthcare settings, including drug treatment programs.
Bachhuber and his colleague expected that the government's new recommendation-HIV screening unless a patient specifically declines-would lead to more widespread testing for HIV in opioid treatment programs. The survey showed that this was not the case, though it did not provide enough information to explain why the opposite trend occurred.
"While it's not entirely clear why for-profit treatment programs are less likely to offer testing, it may help their bottom line," says Bachhuber. "Offering testing is not required by federal and most state regulations, and may not be reimbursed for many patients (for example, those who do not have insurance or have poor coverage). For-profit programs may therefore cut costs and increase profits by not offering testing."
The survey also didn't look at whether patients were referred for off-site screening. However, this would likely have had little impact on the overall testing rate for HIV. In a 2012 study in the American Journal of Public Health, researchers found that only 18 percent of people in drug treatment programs who were referred off-site for HIV screening received their results, compared to more than 80 percent who underwent on-site testing.
With rapid gains now being made in HIV and hepatitis C research and treatments, policy officials must determine how to reverse the decline in these lifesaving screenings in drug treatment programs.
"We are planning a follow-up study," says Bachhuber, "to get at the specific reasons why more treatment programs don't offer testing."
------------------------------------------------------
Research Letter | December 25, 2013
Changes in Testing for Human Immunodeficiency Virus, Sexually Transmitted Infections, and Hepatitis C Virus in Opioid Treatment Programs
Marcus A. Bachhuber, MD1; Chinazo O. Cunningham, MD, MS1 1Division of General Internal Medicine, Albert Einstein College of Medicine, Bronx, New York
JAMA.
Opioid dependence is a risk factor for human immunodeficiency virus (HIV), sexually transmitted infections (STIs), and hepatitis C virus (HCV) infection.1 Opioid treatment programs, which provide treatment to more than 300 000 opioid-dependent individuals in the United States,2 are well-positioned to offer testing for these infectious diseases to a high-risk population. They were among the first venues to offer HIV testing and are more likely to offer HIV, STI, and HCV testing than other drug treatment programs.1 Private for-profit opioid treatment programs are increasingly widespread and such programs offer on-site HIV testing less often than nonprofit and public programs.3 However, with the 2006 national recommendations for routine opt-out HIV testing,4 we hypothesized that the percentage of programs offering on-site testing for HIV, STIs, and HCV would increase.
METHODS
To determine the percentage of opioid treatment programs offering on-site testing over time, we analyzed the National Survey of Substance Abuse Treatment Services.5 The survey is sent to directors of all known drug treatment facilities (response rate, 91.4%-96.5%); survey questions had minor wording changes over time. We tabulated the percentage of opioid treatment programs offering on-site HIV, STI, and HCV testing from 2000 to 2011; there was no survey in 2001. We compared the percentage of for-profit, nonprofit, and public (owned and operated by local, state, tribal, or federal government) programs offering on-site testing over time. Programs with missing data (0.2%-3.9% per year) were excluded. We calculated the relative difference in programs offering on-site testing in 2000 and 2011 using SAS version 9.3 (SAS Institute Inc). P values were calculated using χ2 tests for trend. A 2-sided P².05 was considered significant. Because the survey is publicly available and contains no patient-level data, the Albert Einstein College of Medicine institutional review board determined this study was not considered human research.
RESULTS
The number of US opioid treatment programs increased from 849 in 2000 to 1175 in 2011. The percentage of programs operating as for-profit businesses increased from 43% to 54%, nonprofits decreased from 43% to 36%, and public programs decreased from 14% to 10%. From 2000 to 2011, the absolute number of programs offering testing for HIV, STIs, and HCV increased but the percentage offering on-site testing for HIV declined by 18% (95% CI, 13%-23%; P < .001) and for STIs by 13% (95% CI, 7%-18%; P < .001). There was no significant change for HCV testing (P = .63; Figure 1).
More than 75% of public programs offered on-site testing for each infection, with no significant change over time. Offering on-site HIV testing declined by 20% (95% CI, 10%-29%; P < .001) among for-profit programs and by 11% (95% CI, 6%-17%; P < .001) among nonprofit programs (Figure 2). Offering on-site STI testing declined by 23% (95% CI, 16%-30%; P < .001) in for-profit programs. Offering HCV testing declined by 13% (95% CI, 3%-22%; P = .002) in for-profit programs and increased by 14% (95% CI, 4%-25%; P < .001) in nonprofit programs.
CONCLUSION
The proportion of US opioid treatment programs offering on-site testing for HIV and STIs declined substantially between 2000 and 2011, despite guidelines recommending routine opt-out HIV testing in all health care settings, including substance abuse treatment facilities. Declines were most pronounced in for-profit programs, suggesting that persons enrolled in these programs may be at increased risk for delayed diagnosis and continued transmission of HIV, STIs, and HCV.
This study had limitations. Referral-based testing was not recorded; however, referral-based services often do not translate into patient use.6 Testing for STIs may be limited to syphilis, which is often required by health departments. The survey is cross-sectional with no identifiers linking responses over time. Because patient-level data are not available, patient requests, use, and opting out of testing cannot be determined.
Opioid treatment programs are important venues for offering testing to high-risk individuals. As the number of for-profit opioid treatment programs increases, and the opioid, HIV, and HCV epidemics continue to intersect, further work is needed to understand and reverse declines in offering on-site testing.
REFERENCES
1. Office of Applied Studies, Substance Abuse and Mental Health Services Administration. The N-SSATS Report: infectious disease screening.http://www.oas.samhsa.gov/2k10/227/227DiseaseScreen2k10.htm. Accessibility verified November 15, 2013.
2 Substance Abuse and Mental Health Services Administration. The N-SSATS Report: Trends in the Use of Methadone and Buprenorphine at Substance Abuse Treatment Facilities: 2003 to 2011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
3 Pollack HA, D'Aunno T. HIV testing and counseling in the nation's outpatient substance abuse treatment system, 1995-2005. J Subst Abuse Treat. 2010;38(4):307-316.
4 Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5 Office of Applied Studies, Substance Abuse and Mental Health Services Administration. The DASIS report: the National Survey of Substance Abuse Treatment Services (N-SSATS).http://www.samhsa.gov/data/2k3/NSSATS/NSSATS.pdf. Accessibility verified November 15, 2013.
6 Metsch LR, Feaster DJ, Gooden L, et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health. 2012;102(6):1160-1167.

No comments:
Post a Comment