Liver International
Special Issue: Proceedings of the 7th Paris Hepatitis Conference International Conference of the Management of Patients with Viral Hepatitis, 13–14 January 2014, Paris, France. Guest Editors: Patrick Marcellin and Tarik Asselah. The publication of this supplement was supported by an unrestricted educational grant from Gilead, Janssen Therapeutics, Janssen, Bristol-Myers Squibb, Roche, Boehringer Ingelheim, Merck, AbbVie, Novartis, Idenix and Alios.
Volume 34, Issue Supplement s1, pages 53–59, February 2014
Review Article
You have free access to this content
Valérie Martel-Laferrière1, Douglas T. Dieterich2,*
Article first published online: 23 DEC 2013
DOI: 10.1111/liv.12396
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd
Keywords:
co-infection;direct acting antiviral agents;hepatitis C; HIV
Abstract
Treating hepatitis C virus (HCV) in HIV/HCV co-infected patients is a challenge. Even if the benefits of achieving a sustained virological response are clear, the rates achieved with the combination of pegylated-interferon and ribavirin are disappointing. The addition of direct acting antiviral agents (DAAs) to the treatment of hepatitis C is revolutionizing the treatment of HCV in mono-infected patients. Even if there have not been any agents approved for the treatment of co-infected patients, many studies specifically designed for this population are ongoing. This article reviews available data on the use of DAAs in co-infected patients and the challenges associated with these new drugs.
Hepatitis C virus (HCV) co-infection is common in patients with HIV. Between 25 and 30% of HIV-infected patients are co-infected with HCV. Rates are even higher in populations where injection drug use is the principal mode of transmission of HIV.
Co-infected patients tend to have lower on-treatment sustained virological response (SVR) rates. SVR rates in the first trials of co-infected patients using pegylated-interferon (PEG-IFN) and fixed dose ribavirin (RBV), were 14–29% in genotype 1 and 44–73% in genotypes non-1 patients [1-3]. The use of weight-based RBV improved the rates to 36–38% in genotype 1 and 53–72% in genotype non-1 patients, which are still lower than the rates in mono-infected patients (40–60% in genotype 1 and 70–80% in genotypes 2 and 3 patients) [1, 4]. Nevertheless, co-infected patients can achieve a durable on-treatment SVR [5]. Like in mono-infected patients, the benefits of the reduction in fibrosis, liver decompensation and all causes of mortality are clear [6, 7].
The introduction of direct-acting antiviral agents (DAAs) has significantly improved the treatment outcomes in mono-infected patients [8]. No agents have been approved for co-infected patients. The scope of this article is to review the management of HCV in co-infected patients in the era of DAAs.
Pretreatment considerations
HIV treatment optimization
There are no absolute rules on the optimization of HIV before initiating HCV treatment. Although most co-infected patients who are treated for HCV are on antiretroviral therapy (ARV), this is not essential. In patients with an indication for HIV treatment, ARV should be initiated first with a regimen that does not have any drug–drug interactions with HCV antivirals. Some co-infected patients may present elevated transaminases when ARV are begun and may need to receive HCV treatment first.
Drug interactions
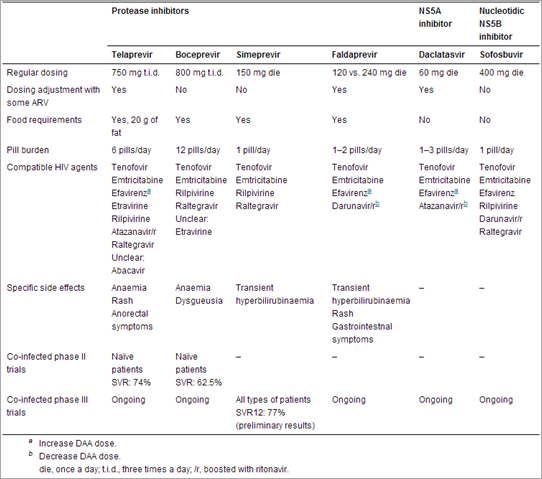
In the era of DAAs, drug–drug interactions are the biggest challenge that healthcare providers face in co-infected patients. For example, in our real-life study with telaprevir, 51.5% of co-infected patients had to change their HIV regimen before initiating HCV treatment to avoid drug–drug interactions [9]. This is often complex for the healthcare provider, especially in multiexperienced patients. A switch in HIV regimen can be emotionally difficult for the patient, who may fear losing control over HIV infection and developing new side effects.
No relevant drug–drug interactions have been found for sofosbuvir [10]. Telaprevir, boceprevir, faldaprevir and daclatasvir all act on the CYP3A [11-14]. Faldaprevir also inhibits CYP2C9 and UGT1A1 [14]. Simeprevir is an inhibitor of intestinal CYP3A and of CYP1A9 [15]. Boceprevir and daclatasvir are p-glycoprotein inhibitors [12, 13].
No clinically significant drug–drug interactions have been identified to date between tenofovir, emtricitabine and DAAs [11-15]. Abacavir is generally considered to be an acceptable choice even if potential interactions involving the UDP-gucuronyltransferase cannot be ruled out for telaprevir. Zidovudine, stavudine and didanosine are contraindicated with PEG-IFN/RBV and are therefore not acceptable options with the PEG-IFN/RBV-containing DAA regimens. Many interactions have been identified with non-nucleoside reverse transcriptase inhibitors. Efavirenz is contraindicated with boceprevir and simeprevir [12, 15]. It can be used with telaprevir, faldaprevir and daclatasvir, but dosages of DAAs must be increased (telaprevir: 1125 mg p.o. t.i.d.; faldaprevir: 240 mg p.o. OD; daclatasvir: 90 mg p.o. OD [15-17]. The integrase inhibitor raltegravir does not interact significantly with the telaprevir, boceprevir or simeprevir [15, 18, 19].
The HIV protease inhibitors (PI) are more complicated. Atazanavir boosted with ritonavir (/r) is considered to be an acceptable combination with telaprevir, while significant interactions have been noted for lopinavir/r, darunavir/r and fosamprenavir/r [20]. Even if they have been used in the phase II trials, PI should not be combined with boceprevir to avoid HIV escape [21]. Boceprevir reduces lopinavir/r, atazanavir/r and darunavir/r concentrations [22]. Although PI were tested in trials on daclatasvir and faldaprevir for co-infected patients, the dose of the DAA had to be reduced in both cases (daclatasvir: 30 mg p.o. OD; faldaprevir: 120 mg p.o. OD) [13, 14]. Darunavir/r is not recommended with simeprevir [15].
Evaluation of cirrhosis
Before initiating HCV treatment, it is important to identify if the patient has cirrhosis. Currently PEG-IFN-based regimens are contraindicated in patients with decompensated cirrhosis. Treatment can be attempted in patients with compensated cirrhosis but the patient's medical condition should be optimized first. Cirrhosis should be managed with regular procedures such as screening for hepatocellular carcinoma and oesophageal varices. Historically, didanosine use, bilirubin above the normal limit and pre-existent cirrhosis have been associated with a higher risk of liver decompensation in the treatment of co-infected patients [23]. The CUPIC study evaluated the outcome of mono-infected patients with cirrhosis treated with telaprevir or boceprevir in an early access program. This study found that low albumin (<35 g/dl) and low platelet count (≤100 000 cells/mm3) were associated with a significantly increased risk of severe on treatment complications or death [24]. Patients at risk of decompensation should be transferred to a liver transplant centre before beginning treatment of HCV. Criteria for liver transplantation in patients with HIV are much stricter than those for patients without, and not all transplant centres offer liver transplantation to patients with HIV.
SVR12 vs. SVR24
The Food and Drug Administration (FDA) has recently accepted a SVR at post-treatment week 12 (SVR12) as a primary outcome for clinical trials instead of the previously used SVR24 [25]. In a large cohort of HCV mono-infected patients treated with PEG-IFN/RBV therapy, it has been demonstrated that 12 weeks post-treatment follow-up appears to be as relevant as 24 weeks to define SVR [26]. Even if the data are limited, this new outcome also seems to be acceptable in co-infected patients. A retrospective analysis of the results of APRICOT and PARADIGM have shown that only 2/941 patients treated with combination IFN or PEG-IFN/RBV relapsed between post-treatment weeks 12 and 24 [27].
Table 1. Direct-acting antiviral agents (DAAs) in phase II or III trials
The phase IIa study VX08-950-110 included 60 co-infected patients naïve to HCV treatment [28]. Thirty-eight patients were in the telaprevir/PEG-IFN/RBV arm and 22 were in the placebo/PEG-IFN/RBV arm. Acceptable HIV regimens for this study were ATZ/r or efavirenz based regimens. If efavirenz was used, the dose of telaprevir was increased to 1125 mg three times daily. SVR rates were 74% in the telaprevir arm and 45% in the placebo arm. Although this is a small study, it is interesting to note that the SVR in the telaprevir arm was significantly higher than the historical SVR rates in co-infected patients with PEG-IFN/RBV and was very similar to the SVR rates observed in phase III telaprevir trials for mono-infected patients (75 and 72%) [32, 33]. As expected, side effects included rash and anorectal symptoms, but there was no statistically significant difference in anaemia. Adverse events were not more frequent in co-infected than in mono-infected patients. Phase III trials in co-infected patients are underway and results are expected in 2014 (NCT01513941 and NCT01467479) [31]. Real-life study results are now available for telaprevir-based therapy in co-infected patients. Cachay et al. has reported that 50% of 24 consecutive co-infected patients treated with telaprevir achieved a SVR [34]. In our study of 33 consecutive co-infected patients treated at Mount Sinai Medical Centre or Johns Hopkins University, 60.6% of patients achieved a SVR [9]. Previous non-responders were included in both cases, explaining in part the lower rate of SVR compared with the clinical trial. Nevertheless, SVR rates were clearly higher than those seen in PEG-IFN/RBV trials.
Careful management of mono- and co-infected patients on telaprevir-based therapy is mandatory to avoid, or at least to limit, severe adverse events. Since the approval of telaprevir, the FDA has modified the package insert to add medical visits to monitor haemoglobin and has added a black-box warning noting the association of telaprevir and cases of Stevens–Johnson syndrome [35]. In the study by Cachay et al., 50% of the patients developed grade IV adverse events according to the Division of AIDS criteria and 29% discontinued therapy because of side effects [34]. In our experience, 24.2% of our co-infected patients discontinued treatment because of side effects, but this was not statistically different than the discontinuation rate in mono-infected patients (13.8%, P = 0.18) [9]. Similarly, hospitalization rates (24.4% vs. 15.5%, P = 0.24) and severe anaemia (45.5% vs. 58.6%, P = 0.18) were elevated, but were not higher in co-infected than in mono-infected patients.
Boceprevir
Like telaprevir, boceprevir is an NS3/NS4A protease inhibitor with activity against HCV genotype 1. It is administered three times per day with food but with no fat requirements. The boceprevir treatment schedule is more complex than the telaprevir schedule with treatment varying from 28 to 48 weeks depending on the patient's previous response to PEG-IFN/RBV, his/her current response to the boceprevir-based regimen and the presence or absence of cirrhosis. There is an initial lead-in phase of 4 weeks with PEG-IFN/RBV before beginning boceprevir in all patients. The most common side effects are anaemia and dysgueusia.
The phase IIa boceprevir trial for co-infected patients was designed for naïve patients [21]. Sixty-four patients were enrolled in the boceprevir group and 34 in the placebo group. After a 4-week PEG-IFN/RBV lead-in, boceprevir or placebo was added for 44 weeks. Patients in the placebo group who failed at week 24 were offered boceprevir/PEG-IFN/RBV for an additional 44 weeks (crossover). Patients receiving didanosine, zidovudine or a non-nucleoside reverse transcriptase inhibitor were excluded. The SVR in the boceprevir group was significantly better than in the placebo group (62.5% vs. 29.4%, respectively, P < 0.01). Four patients were included in the crossover arm and three achieved SVR. Although almost all patients in both arms presented with treatment related adverse events, adverse events by symptom were more common in the boceprevir arm. On the other hand, there was no statistically significant difference in the rate of serious adverse events and treatment discontinuations because of adverse events between the two groups. Seven patients had an on-treatment HIV breakthrough, three (5%) in the boceprevir arm and four (12%) in the placebo arm. All these patients were on a HIV PI-based regimen, but this was also true for 86% of the boceprevir group and 91% of the placebo group. A phase III trial, sponsored by the National Institute of Allergy and Infectious Diseases, is underway [31].
Simeprevir
Simeprevir will probably be the next PI approved by the FDA because the New Drug Application of this agent has already been submitted[36]. The administration schedule of simeprevir is similar to that for telaprevir: triple therapy in combination with PEG-IFN/RBV followed by PEG-IFN/RBV alone for 24–48 weeks. Compared with boceprevir and telaprevir, this agent has a broader antiviral activity by genotype (genotypes 1 and 2, 4–6) and seems to be associated with fewer side effects (only transient hyperbilirubinaemia) [37].
Preliminary results of the C212 study were presented at the 2013 Conference on Retroviruses and Opportunistic Infections (CROI) [29]. This phase III study was designed for co-infected patients with any type of previous treatment response. All patients received 12 weeks of simeprevir. Naïve or relapser patients without cirrhosis were treated with PEG-IFN/RBV for 24 vs. 48 weeks depending on the virological response while patients with cirrhosis and previous partial or non-responders received 48 weeks of PEG-IFN/RBV. The ARVs abacavir, tenofovir, emtricitabine, lamivudine, raltegravir, rilpivirine, maraviroc and enfuvirtide were allowed. Overall, 106 patients were enrolled in this study. On-treatment week 4 results are available for 104: 66% of the patients were undetectable. As seen in many mono-infected trials, relapse patients had the best response rates (93%) while the rate in non-responders was 37%. SVR4 and SVR12 rates were presented for the subset of patients eligible for 24-week of treatment. Eighty-six percent of these 35 patients achieved SVR4 (naïve: 84%, relapsers: 90%) and 77% achieved SVR12 (naïve: 75%, relapsers: 80%). No HIV virological failure was reported.
Faldaprevir
Faldaprevir is the fourth PI to provide available data in co-infected patients. The optimal dosing and duration of therapy are still under evaluation in the phase III program (STARTVerso 1–4) [31]. The most common side effects associated with faldaprevir are gastrointestinal symptoms, rash and hyperbilirubinaemia [38].
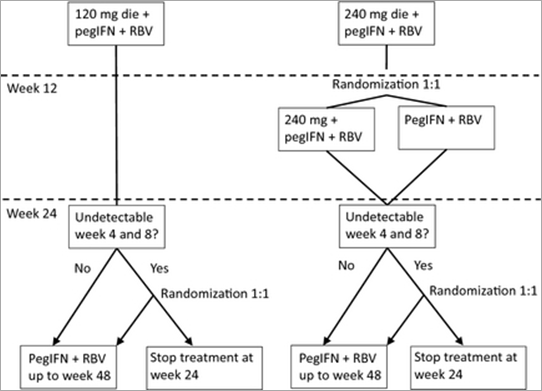
STARTVerso 4 is the phase III program testing faldaprevir in co-infected patients [30]. This trial included naïve and relapse patients. Acceptable HIV non-nucleoside reverse transcriptase inhibitors were raltegravir, maraviroc, efavirenz, atazanavir/r and darunavir/r. Two doses were tested in this study (120 and 240 mg). To manage potential drug–drug interactions, patients receiving efavirenz were assigned to the 240 mg group while patients receiving darunavir/r or atazanavir were assigned to the 120 mg group. Patients receiving maraviroc or raltegravir were randomized. A total of 308 patients were included in this study, including 17% with cirrhosis. This study design of STARTverso 4 is complex and is presented in Fig. 1. Preliminary on-treatment results were presented at CROI 2013. At week 12 of treatment, 82% of the previously naïve patients and 91% of the previous relapsers had undetectable viral loads.
Figure 1. STARTverso 4 design. pegIFN, pegylated-interferon; RBV, ribavirin; die, once a day.
Of interest, faldaprevir has been tested in combination with deleobuvir, a non-nucleosidic NS5B inhibitor, and RBV for the treatment of HCV mono-infected patients [39]. There were five arms in this phase IIb trial to explore the impact of the length of treatment, the use of ribavirin and the administration schedule (two vs. three times daily) of deleobuvir. SVR was achieved by 52–69% of the patients [39]. The combination is currently being studied in co-infected patients (NCT01525628) [31].
Daclatasvir
Daclatasvir is an NS5A inhibitor with activity against HCV genotype 1. It is administered once daily at a dose of 60 mg, which may be adjusted to 30 or 90 mg when combined with an HIV protease inhibitor or efavirenz respectively. Daclatasvir has been studied in combination with many agents including PEG-IFN, RBV, sofosbuvir and asunaprevir. A phase III trial in co-infected patients is ongoing (NCT01471574) [31]. It was designed to include HCV genotype 1 naïve patients. Daclatasvir will be administered for 24 weeks and PEG-IFN/RBV for 24 vs. 48 weeks depending on virological response criteria for shorter therapy.
Sofosbuvir
Sofosbuvir is a nucleotide NS5B polymerase inhibitor [40]. It is administered once daily at a dose of 400 mg. Sofosbuvir is a pangenotypic agent and no specific side effects have been identified. It will probably be the first non-PI DAA approved because its New Drug Application Form was submitted to the FDA in the Spring of 2013 [41]. Mono-infected patients with HCV genotypes 1, 4–6, receive sofosbuvir in combination with PEG-IFN and RBV for 12 weeks [40]. Patients with genotypes 2 and 3 receive an interferon-free combination with RBV alone [40, 42]. Two phase III trials (NCT01667731 and NCT01783678) are ongoing [31]. One study includes patients with genotypes 1–3 and the other 1–4. Interestingly, these two studies are interferon-free even in patients with genotype 1 or 4.
Acute hepatitis C and DAAs
Because of the development of new drugs for the treatment of chronic HCV, little attention is being paid to acute hepatitis C. Nevertheless, this condition is still a major health issue, especially in patients with HIV. Sexual transmission of HCV in HIV-infected patients is now well-recognized, with outbreaks described worldwide.
The European AIDS Treatment Network (NEAT) currently recommends the use of PEG-IFN and weight-based RBV for the treatment of acute HCV in HIV-infected patients. Twenty-four weeks of treatment is recommended for patients who achieve a rapid virological response (undetectability at week 4; RVR). Other patients should be treated for 48 weeks and patients who do not present a 2 log10 drop at week 12 should discontinue treatment because the probably will not achieve a SVR. NEAT recommends beginning treatment in patients with persistent HCV RNA 12 weeks after diagnosis or if a 2 log10 decrease in HCV RNA does not occur 4 weeks after the initial HCV RNA [43]. The EASL and AASLD do not have specific guidelines for acute HCV in co-infected patients.
As expected from results in acute vs. chronic monoinfected patients, co-infected patients with acute HCV tend to have better SVR rates than those with chronic infection. Rates in published trials vary from 53 to 82% [44, 45]. Very little information is available on DAAs in the treatment of acute HCV. Fierer presented the preliminary results of a small study with telaprevir at CROI 2013. Eighty-five per cent (17/20) patients treated with 12 weeks of PEG-IFN/RBV and telaprevir achieved SVR4 [46].
Conclusions
Studies clearly show that DAAs will change the face of treatment in patients with HIV/HCV coinfection. These new agents seem to fill in the gap in treatment outcomes between co- and mono-infected patients. Adverse events with telaprevir and boceprevir must be managed, but do not appear to be more important in co-infected patients and side effect profiles, at least for haematological effects will probably be less severe.
Drug–drug interactions are a real issue. Compatible options of ARV and antiviral combinations are limited. This review only discussed trials on co-infection with available or soon to be available data. In the up-coming era of an interferon-free regimen, treatment will probably combine many DAA's, increasing the chance of drug–drug interactions.
Another potential issue in the management of co-infected patients will be access to treatment. Specific approval by agencies such as the FDA, European Medicine Agency or Health Canada for this population will be required to insure reimbursement of drugs by insurance companies.
Disclosure
Valérie Martel-Laferrière has nothing to disclose. Douglas T. Dieterich serves as a paid lecturer, consultant and for his service on scientific advisory boards for Gilead Sciences, Boehringer Ingelheim, Novartis, Vertex Pharmaceuticals, Achillion, Tibotec, Merck, Genentech and Hoffman-La Roche, Inc. and Bristol-Myers Squibb.
No comments:
Post a Comment