Topics in Antiviral Medicine Volume 21 Issue 5 December 2013/January 2014
Perspective
Cure of hepatitis C virus (HCV) infection is achievable without interferon alfa through the use of new direct-acting antiviral (DAA) drugs. In this era of interferon alfa–sparing therapy, however, interferon alfa sensitivity still matters, even as it turns out, if interferon alfa is not used. Inclusion of ribavirin in the treatment regimen remains a factor in treatment response, as does duration of treatment. HCV genotype and subtype remain relevant considerations in choosing a treatment regimen, and viral resistance may emerge when treatment fails. The potency and barrier to resistance of new DAAs and the use of appropriately designed interferon alfa–sparing combinations can overcome obstacles to cure posed by HCV resistance, interferon alfa resistance, and differences in response based on HCV genotype and subtype. Studies demonstrating the use of new DAAs to overcome these obstacles are discussed. This article summarizes a presentation by David L. Thomas, MD, MPH, at the IAS–USA continuing education program held in New York, New York, in June 2013.
Keywords: HCV, hepatitis C, treatment, interferon, sparing, interferon alfa–sparing, direct-acting antivirals, DAAs, genotype, protease inhibitor, NS5A, NS5B, resistance, sofosbuvir, simeprevir
The first hepatitis C virus (HCV)-infected patients were cured in 1984 and 1985, before it was known that a virus was causing their disease. In studies at the National Institutes of Health (NIH), Hoofnagle and colleagues used interferon alfa to treat individuals with non-A or non-B hepatitis and observed a normalization of liver enzymes in these individuals.1 After the virus was identified, it became clear that eradication of HCV had also been achieved. Since then, interferon alfa has been the mainstay in the treatment and cure of HCV infection. Regimens that include interferon alfa have been associated with marked improvement in important therapeutic outcomes, including reductions in mortality, liver cancer, and other serious liver-related events. However, interferon alfa–based treatments are not always successful and have been associated with considerable toxicity and morbidity. Now, a new era of interferon alfa–sparing HCV treatment has begun, and soon it will be possible to achieve cure for most patients with as little as one pill once a day.
The Context
There are viral replication foci in approximately 10% to 20% of hepatocytes distributed throughout an HCVinfected liver. Approximately 1 to 50 viruses can be detected at any one time in a single hepatocyte, but there are places in the liver with no detectable HCV RNA. Thus, to be effective, HCV treatment must reach all sites in the liver that harbor infected hepatocytes. Further, in an HCV-infected liver, there are approximately 1 trillion viruses produced every day. If an environment becomes inhibitory for one variant, another will quickly take its place. To be successful, HCV treatment must also overcome the genetic and phenotypic heterogeneity of HCV. The ongoing host immune response to the virus sometimes clears infection. When it does not, those patients with chronic infection seem to have 1 of 2 different states. One state is characterized by inflammation and the upregulation of interferon-stimulated genes (ISGs). The HCV infections of persons with this high-ISG state are less responsive to exogenous interferon alfa, and these individuals often have a haplotype around the genes for the lambda interferons. In particular, the commercially available IL28B test to determine specific genotype indicates that individuals with a high-ISG state HCV infection have what is called the unfavorable IL28B T allele genotype. Persons with the other state have low expression of ISGs, and their HCV infections remain responsive to exogenous alpha-interferon. They are much more likely to have different genotypes near IL28B, and in particular, the favorable IL28B C allele genotype. Interestingly, persons in these 2 states of differing interferon sensitivity and the associated IL28B genotypes continue to have different susceptibilities to interferonsparing treatments, reflecting their intrinsic tendency to eradicate infection.
The context of infection can also be important when liver tissue is markedly distorted by cirrhosis. Patients with cirrhosis are harder to treat effectively, even with new interferon alfa–sparing regimens, possibly because the distorted tissue inhibits drug penetration or because of factors such as changes in portal pressure.
The Tools
Almost all of the major steps in the HCV life cycle are potential targets for inhibiting viral replication, including entry, endosomal release and internal ribosome entry site (IRES)-dependent translation, protease cleavages, membranous web formation and lipoprotein assembly linked to nonstructural protein (NS) 5A (NS5A), and NS5B RNAdependent RNA polymerase (RdRp) activity. Investigational drugs have been developed to target nearly all of these steps but several drugs are further along in development.
Currently, the 3 main groups of HCV drugs are protease inhibitors (PIs; ie, telaprevir and boceprevir), NS5Aacting agents, and nucleos(t)ide RdRp inhibitors. HCV PIs are potent, can affect a variable range of HCV genotypes, and have a variable barrier to resistance, especially with HCV genotype 1a compared with genotype 1b. Nucleos(t)ide polymerase inhibitors are characterized by high potency, can affect a wide range of HCV genotypes, and have a high barrier to resistance. NS5A-targeting drugs also have high potency, but their barrier to resistance has been problematic.
In formulating approaches to combination therapy, the strategy is to exploit the beneficial properties of the different drug groups and also to include a drug with a high barrier to resistance. In some cases, the investigation of drug combinations is limited by the practicalities of drug development, with drug companies developing combinations of their own products. Thus, some of the most obvious drug combinations for HCV therapy are not being tested in phase III trials.
Interferon Alfa–Sparing Treatment
Although there are data to show that HCV can be cured with investigational treatments that do not include interferon alfa, sensitivity to interferon alfa may still be a factor in treatment response and resistance may emerge when a treatment is unsuccessful. HCV genotype and subtype, ribavirin use, and duration of treatment may also be factors in the efficacy of HCV therapy. However, a drug’s potency or its barrier to resistance can trump these other factors.
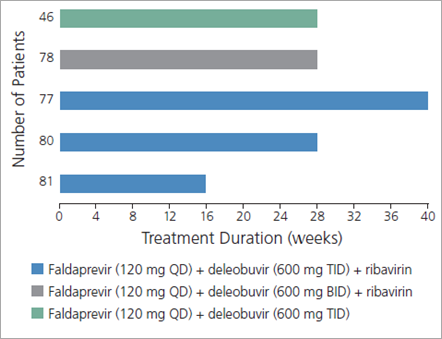
Figure 1. Design of a trial evaluating the use of the investigational drugs faldaprevir and deleobuvir, with or without ribavirin, in 362 treatment-naive patients with genotype IL28B (CC or non-CC) and hepatitis C virus (HCV) genotype 1 (subtype 1a or 1b) infection. Thirty-three patients enrolled in the trial had compensated cirrhosis. BID indicates twice daily; QD, once daily; TID, thrice daily. Adapted with permission from Zeuzem et al.2
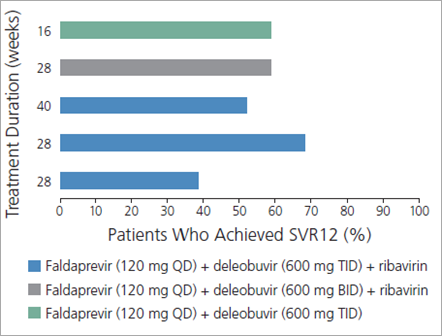
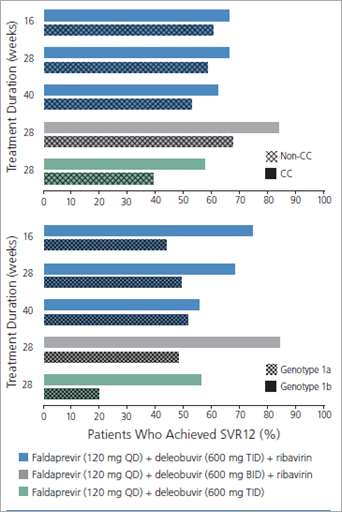
A study assessing the use of the investigational HCV PI faldaprevir (once daily) combined with the investigational nonnucleoside (nn) NS5B inhibitor deleobuvir (formerly BI 207127; twice or thrice daily), with or without ribavirin, for various treatment durations in treatment-naive patients with HCV genotype 1 infection illustrates many of these points (Figure 1).2,3 Substantial rates of sustained virologic response (SVR) 12 weeks after cessation of treatment (SVR12), considered to constitute cure, were observed with all regimens, indicating that cure of HCV infection is indeed possible without the use of interferon alfa (Figure 2).2 SVR12 rates were higher in the groups that received ribavirin, indicating that ribavirin may still impact the efficacy of HCV treatment. In the same study, IL28B genotype and interferon alfa sensitivity were still factors in treatment response, as shown by the higher SVR12 rates seen in patients with the IL28B CC genotype, regardless of which regimen they received (Figure 3, top).2 Furthermore, HCV subtype remained a factor, as shown by the higher SVR12 rates in patients with HCV genotype 1b versus genotype 1a infection, again regardless of which regimen they received (Figure 3, bottom).2 Several PIs show marked differences in their ability to eradicate genotype 1a virus compared with genotype 1b virus. In this study, the SVR12 rate in patients with HCV genotype 1a infection whose regimens did not include ribavirin was only 11%.2 Faldaprevir may be indicated for use only in patients with HCV genotype 1b infection.
Figure 2. Sustained virologic response (SVR) rates, by treatment group, in a trial evaluating the use of the investigational drugs faldaprevir and deleobuvir, with or without ribavirin, in treatment-naive patients with hepatitis C virus (HCV) genotype 1 infection. BID indicates twice daily; QD, once daily; SVR12, SVR 12 weeks after cessation of treatment; TID, thrice daily. Adapted with permission from Zeuzem et al.2
Figure 3. Sustained virologic response (SVR) rates, by treatment group, in a trial evaluating the use of the investigational drugs faldaprevir and deleobuvir, with or without ribavirin, in treatment-naive patients with genotype IL28B (CC or non-CC) and hepatitis C virus (HCV) genotype 1 (subtype 1a or 1b) infection, stratified by patient genotype (top) and HCV subtype (bottom). BID indicates twice daily; QD, once daily; SVR12, SVR 12 weeks after cessation of treatment; TID, thrice daily. Adapted with permission from Zeuzem et al.2
In another study, 19 treatmentnaive patients with HCV genotype 1 infection received a 12-week regimen of the ritonavir-boosted investigational PI ABT-450, the investigational nnNS5B inhibitor ABT-333, and ribavirin.4 Of these 19 patients, 47% had IL28B T genotype and 89% had HCV genotype 1a infection. All 19 patients achieved virologic suppression; SVR12 was achieved in 18 patients (95%), with 1 patient withdrawing from the study due to elevated levels of alanine transaminase. However, when the same regimen was given to 17 of the study patients whose previous regimen of interferon alfa and ribavirin had failed (all patients had IL28B T genotype and 94% of patients had HCV genotype 1a infection), SVR12 was achieved in only 8 patients (47%), 6 experienced breakthrough viremia, and 3 relapsed. Of the latter 9 patients, viral resistance developed in all but 1.
In a separate study, the same regimen was fortified with the investigational NS5A inhibitor ABT-267, which is highly potent but does not have a high barrier to resistance.5,6 As shown in Figure 4, SVR rates in patients with prior nonresponse to interferon alfa were 89% to 98% when ABT-267 was added to their regimen, with very few patients experiencing breakthrough or relapse. This study demonstrates that the addition of another drug can dramatically improve response rates by overcoming resistance to interferon alfa in those with prior nonresponse.
(Click to view larger image)
Figure 4. Sustained virologic response (SVR) rates, by treatment group, in a trial evaluating the use of the ritonavir-boosted (/r) investigational protease inhibitor ABT-450, the investigational nonstructural protein (NS) 5A (NS5A) inhibitor ABT-267, the investigational nonnucleoside NS5B inhibitor ABT-333, and ribavirin in 438 hepatitis C virus (HCV)-infected, treatment-naive patients and in 133 HCV-infected patients with prior nonresponse to interferon alfa. SVR12 indicates SVR 12 weeks after cessation of treatment; SVR24, SVR 24 weeks after cessation of treatment. Adapted with permission from Kowdley et al.6
Similar principles of treatment may be observed when a nucleotide inhibitor (ie, sofosbuvir) with broad genotypic activity is used with or without ribavirin in individuals with HCV genotype 2 or 3 infection. Sofosbuvir has been approved by the US Food and Drug Administration (FDA) since the June presentation summarized here.
In an initial study of sofosbuvir given with or without ribavirin to treatment-naive patients with HCV genotype 2 or 3 infection, the SVR24 rate was 100% (10 of 10) in patients who received sofosbuvir and ribavirin and 60% (6 of 10) in patients who received sofosbuvir monotherapy.7,8 Likewise, in 25 treatmentnaive patients with HCV genotype 1 infection who received a combination of sofosbuvir and ribavirin, the SVR24 rate was 84%. However, SVR24 was achieved in only 1 of 10 HCV genotype 1–infected patients with prior nonresponse to interferon alfa. Therefore, the use of sofosbuvir and ribavirin can achieve high SVR rates in treatment- naive patients who are relatively sensitive to interferon alfa, whether they have HCV genotype 1, 2, or 3 infection. However, as represented in patients with prior nonresponse, resistance to interferon alfa still plays a role and, as discussed below, it can be overcome.
A study of sofosbuvir and ribavirin in 201 patients with prior nonresponse and HCV genotype 2 or 3 infection demonstrated that genotype and duration of treatment were factors in achieving SVR.7 Among patients with HCV genotype 2 infection, SVR was achieved in 94% of those who received 16 weeks of treatment and in 86% of those who received 12 weeks of treatment. Among patients with HCV genotype 3 infection, SVR rates were lower but still reflected a benefit with longer treatment duration: SVR was achieved in 62% of the 16-week treatment group and 30% of the 12-week treatment group. Longer duration of treatment was also beneficial to patients with cirrhosis: SVR was achieved in 66% of the 16-week treatment group and 31% of the 12-week treatment group.9
In another study, the addition of the investigational NS5A-acting agent ledipasvir to a 12-week regimen of sofosbuvir and ribavirin produced SVR in 100% (9 of 9) of HCV genotype 1–infected patients with prior nonresponse and in 100% (25 of 25) of treatment- naive, HCV genotype 1–infected patients.10 Sofosbuvir and ledipasvir, coformulated as a single, once-daily pill, taken with or without ribavirin for a 12- or 16-week treatment period is currently being assessed in phase III trials.
Early phase studies have evaluated investigational HCV drugs from 2 different pharmaceutical companies. Although combination forms of these drugs do not seem destined for FDA approval anytime soon, there is some preliminary evidence that the treatments are effective. FDA approval of some of these individual drugs may offer clinicians the possibility of devising interferon alfa–sparing regimens in the very near future and prior to FDA approval of combination regimens.
For instance, simeprevir was recently approved by the FDA for use in combination with ribavirin and interferon alfa in HCV genotype 1–infected patients with compensated liver disease, including cirrhosis. And an early phase study has shown that the investigational combination of the PI simeprevir and the nucleotide inhibitor sofosbuvir, with or without ribavirin, produced SVR in more than 90% of HCV genotype 1–infected patients with prior nonresponse.11 Similarly, the combination of the investigational NS5A-acting agent daclatasvir and the nucleotide inhibitor sofosbuvir, with or without the addition of ribavirin, produced an SVR rate of nearly 100% in a study of 41 patients whose prior treatment with telaprevir, boceprevir, and interferon alfa plus ribavirin had failed.12
Conclusion
Interferon alfa–sparing treatment for HCV infection is coming. Presently, many practitioners are faced with the decision of whether to treat their patients with currently available drugs or to wait until new drugs are available. As availability of the newer options approaches, many practitioners are choosing to wait or are enrolling their patients in clinical trials that evaluate new treatments.
With the recent approval by the FDA of sofosbuvir in combination with ribavirin for patients with HCV genotype 2 or 3 infection and in triple therapy with interferon alfa and ribavirin for treatment-naive patients with HCV genotype 1 or 4 infection, waiting to begin treatment may no longer be necessary for some patients. The recent FDA approval of simeprevir to be used with peginterferon alfa and ribavirin for patients with HCV genotype 1 infection also makes possible the use of sofosbuvir in combination with simeprevir, but this combination is investigational and not approved by the FDA at this time. The combination was effective for some patients in the COSMOS (Combination of Simeprevir and Sofosbuvir in HCV Genotype 1 Infected Patients) study.11 In 2014, approvals of additional medications, including interferon alfa–sparing combination regimens for HCV genotype 1, are likely.
More data are needed to determine the best ways to use new treatments for HIV/HCV-coinfected patients and other special populations. Substantial drugdrug interactions between sofosbuvir and most antiretroviral drugs have not been observed, and sofosbuvir and ribavirin regimens are already being studied in HIV/HCV-coinfected patients.
Interferon alfa may still be used to treat HCV infection for the foreseeable future in settings where new treatments may initially be too expensive. However, the widespread use of interferon alfa–sparing treatment options for all HCV-infected patients, whether in resource-rich or resource-poor locales, can be envisioned.
Presented by Dr Thomas in June 2013. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Thomas in December 2013.
Financial Affiliations: Dr Thomas has received grants awarded to his institution from Gilead Sciences, Inc, and Merck & Co, Inc.
Additional Suggested Reading
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Eng J Med. 2013;368(20):1878-1887.
References
1. Hoofnagle JH, Mullen KD, Jones DB, et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315(25):1575-1578.
2. Zeuzem S, Soriano V, Asselah T, et al. Interferon (IFN)-free combination treatment with the HCV NS3/4A protease inhibitor BI 201335 and the non-nucleoside NS5B inhibitor BI 207127 +/- ribavirin (R): final results of the SOUND-C2 and predictors of response [Abstract 232]. 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 9-13, 2012; Boston, Massachusetts.
3. Zeuzem S, Soriano V, Asselah T, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369(7):630-639.
4. Poordad F, Lawitz E, Kowdley KV, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368(1):45-53.
5. Kowdley K, Lawitz E, Poordad F, et al. A 12- week interferon-free treatment regimen with ABT-450/r, ABT-267, ABT-333 and ribavirin achieves SVR12 rates (observed data) of 99% in treatment-naive patients and 93% in prior null responders with HCV genotype 1 infection. 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). November 9-13, 2012; Boston, Massachusetts.
6. Kowdley K, Lawitz E, Poordad F, et al. Safety and efficacy of interferon-free regimens of ABT-450/r, ABT-267, ABT-333 +/- ribavirin in patients with chronic HCV GT1 infection: results from the AVIATOR study. 48th Annual Meeting of the European Association for the Study of the Liver. April 24-28, 2013; Amsterdam, The Netherlands.
7. Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368(1):34-44.
8. Gane E, Stedman C, Anderson J, et al. 100% Rapid virologic response for PSI- 7977 + ribavirin in genotype 1 null responders (ELECTRON): early viral decline similar to that observed in genotype 1 and genotype 2/3 treatment-naive patients. 19th Conference on Retroviruses and Opportunistic Infections (CROI). March 5-8, 2012; Seattle, WA.
9. Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867- 1877.
10. Gane E, Hyland R, Ding X, et al. ELECTRON: 100% suppression of viral load through 4 weeks post-treatment for sofosbuvir + ledipasvir (GS-5885) + ribavirin for 12 weeks in treatment-naive and -experienced hepatitis C virus GT1 patients [Abstract 41LB]. 20th Conference on Retroviruses and Opportunistic Infections (CROI). March 3-6, 2013; Atlanta, Georgia.
11. Lawitz E, Ghalib R, Rodriguez-Torres M, et al. Suppression of viral load through 4 weeks post-treatment results of a oncedaily regimen of simeprevir + sofosbuvir virus GT1 null responders [Abstract 155LB]. 20th Conference on Retroviruses and Opportunistic Infections (CROI). March 3-6, 2013; Atlanta, Georgia.
12. Sulkowski M, Gardiner DF, Rodriguez- Torres M, et al. Sustained virologic response with daclatasvir plus sofosbuvir ± ribavirin (RBV) in chronic HCV genotype (GT) 1–infected patients who previously failed telaprevir (TVR) or boceprevir (BOC). 20th Conference on Retroviruses and Opportunistic Infections (CROI). April 24-28, 2013; Amsterdam, The Netherlands.
Top Antivir Med. 2014;21(5):152-156
©2014, IAS–USA. All rights reserved

No comments:
Post a Comment